Szakmai irányelv / Consensus Guideline
Diagnosis and antiviral therapy of hepatitis B and D – Hungarian Consensus Guideline
Summary
Diagnosis and treatment of HBV±HDV infection means for the patient to be able to maintain working capacity, to increase quality of life, to prevent cancer, and to prolong life expectancy, while society benefits from eliminating the chances of further transmission of the viruses, and decreasing the incidence and overall costs of serious complications. The guidelines outline the treatment algorithms that have been in place since 2019, developed at a consensus meeting of physicians involved in treating these diseases. The indications of treatment is based upon viral examinations (including viral nucleic acid determination) with determinations of disease activity and stage of related liver disease (by laboratory tests, pathologic, and/or non-invasive methods, i.e., elastographies or biochemical scores), and excluding contraindications. The first choices of therapy in chronic hepatitis B infection includes pegylated interferon for 48 weeks or continuous nucleotide/nucleoside analogue (NAs: entecavir or tenofovir). NAs must be continued for at least 12 months after hepatitis B surface antigen seroconversion. Some NAs are not available/not routinely used in Hungary. Lamivudine is no longer a first choice; patients currently taking lamivudine must switch if response is inadequate. Appropriate treatment of patients taking immunosuppressive medications is highly recommended. Pegylated interferon based therapy is recommended for the treatment of concomitant hepatitis D infection.
Introduction
Hepatitis B virus is a member of the hepadnavirus family with a circular DNA genome. Among adult patients, acute HBV infection becomes chronic in 90%. Chronic HBV infection usually causes asymptomatic infection for decades, during which, the patient can infect other people. However it can also cause chronic inflammation in the liver (CHB) and – by time – severe liver diseases (cirrhosis, liver cancer) which reduces the ability to work, the quality of life, and also the life expectancy. In addition, treatment of complications requires great cumulative costs from the healthcare system. HBV is a direct oncogene. Therefore every patient with chronic HBV infection has an increased risk of developing liver cancer even if the HBV infection doesn’t cause any significant liver disease. The estimated seroprevalence of HBV infection (HBsAg+) in Hungary is 0,3–0,5%.
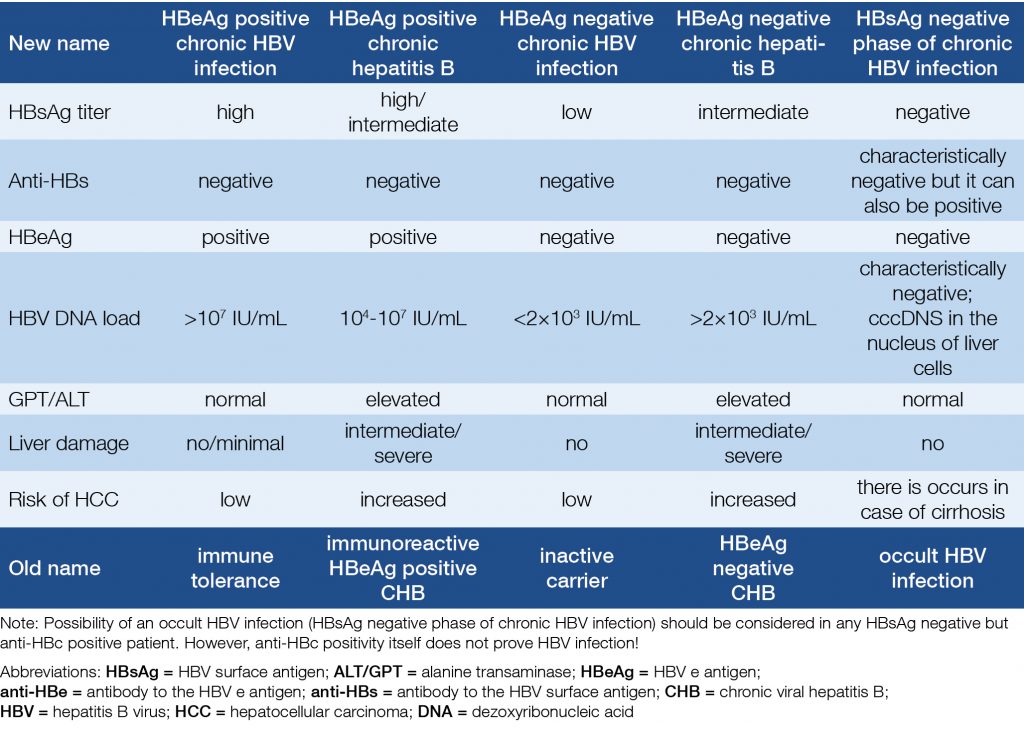
A new nomenclature of the five phases of the HBV infection was accepted by the EASL in 2017. Each phase is differentiated from the others on the basis of HBeAg status, degree of replication (HBV DNA), biochemical activity (GPT/ALT), and the inflammation in the tissues of the liver (if histology performed) (Table 1).

Table 1. New terminology of phases of chronic HBV infection (3)
Hepatitis D (delta) virus contains RNA genome. It’s a defective virus; its replication depends on the presence of HBV infection. Thus, it can infect only patients with HBV or it can be concomitant infection. In most cases, HDV infection causes more severe liver damage and/or faster progression of an existing disease (leading to cirrhosis) than HBV alone. HDV also means a higher risk of liver cancer.
Chronic HBV/HDV infections usually do not cause any symptoms. However, early diagnosis and treatment of the HBV/HDV infection are essential. From the patients’ aspect: it helps the patient to maintain their ability to work, improves the quality of life, helps prevention of cancer, and extends the disease-free lifespan. From the aspect of the environment of the patient and also the society: it stops the transmission of the infection and reduces the costs of the healthcare system (because of the prevention of severe liver diseases). In Hungary, gastroenterologists, infectologists and tropical diseases specialists are assigned to manage HBV/HDV infections, according to consensus-based professional guideline (since 2006), updated annually to include new evidences, publications, international recommendations, approved labels of drugs and cost-benefit analysis (1, 2, 3, 4, 5). However, IFN-based therapies for HBV and HDV (just like therapies for HCV) are only authorized for specialists at designated Hepatitis Centers, under the supervision of a Hepatitis Therapy Committee (former: Interferon Committee, hereinafter referred to Committee; office: H-1092 Budapest Kinizsi u. 22., E-mail: gastroent@gmail.com, web: www.gastroent.hu). Members of the Committee are delegated by professional organizations.
This current guideline is an updated version of the previous from 2017 (1). It was accepted on the 20th of September, 2019. However, the latest financing protocol of NEAK (based on a previous professional guideline from 2010) was accepted in 2010 and published in the official publication of Ministry of Health in 2011 (2). Members of the Committee were also involved in development of this financial protocol.
The aim of this guideline is to update previous recommendations from 2017. In this guideline, references can be found only for recommendations that are different from the once in the 2017 version or not included in it. This guideline doesn’t substitute the directions of the regulations of drug labels published by the OGYÉI!
Diagnosis
Confirmation of HBV/HDV infections and related liver disease
Confirmation of HBV infection, HBV genotyping
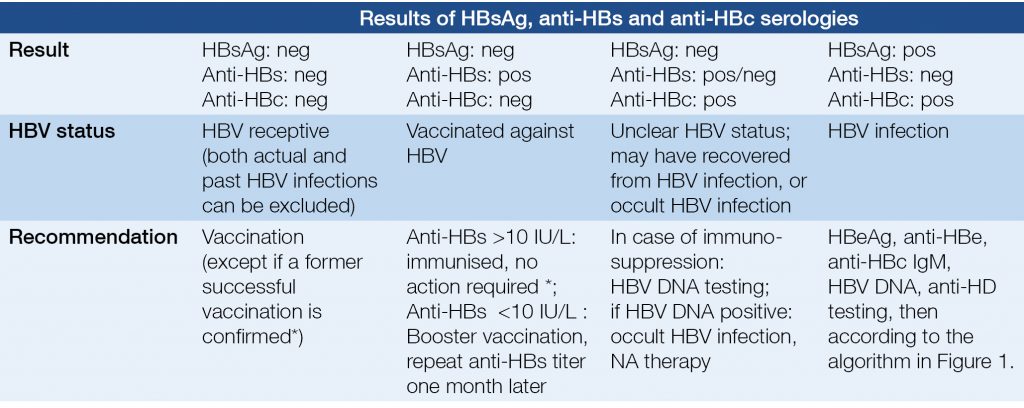
For all patients with liver disease and unknown HBV and/or HCV status, HBsAg, anti-HBs, anti-HBc (and anti-HCV) serologies are recommended (Table 2). HBV vaccination is recommended if HBsAg and anti-HBs serologies are negative. HBsAg negativity and anti-HBc positivity must be documented in the medical record of the patient, because occult HBV infection is of great importance in blood donation and might be relevant in certain diseases and therapies.
Detection of HBsAg and/or HBeAg, and/or HBcAg, and/or HBV DNA confirms HBV infection. If persists for more than 6 months, confirms chronic HBV infection. Replication of HBV is to be confirmed by quantitative PCR for HBV DNA. It is recommended even if HBsAg and anti-HBs negative, but anti-HBc positive, and liver disease is suspected. Therapy-related PCR must be performed in laboratories working with ISO qualification and continuously take part in quality control, according to the following methodological and quality terms:
- real-time PCR with properly sensitive tests (qualification: CE-IVD),
- low detection limit of HBV DNA: lower limit of detection ≤20 IU/mL,
- linear quantification range of HBV DNA: 20 IU/mL – 108 IU/mL,
- PCR results necessary to the treatment decisions must be ready in 5 working days after the arrival of the sample to the laboratory.
If possible, HBV genotyping is recommended. A and B genotypes of HBV respond better to IFN therapy than other genotypes.
Table 2. Serological diagnosis of HBV infection; evaluation HBV status and recommendations
Confirmation of HDV infection
All HBsAg positive patients should be screened for anti-HD. If anti-HD is positive a positive anti HD-IgM and/or HDV RNA and/or HDAg confirms active HDV infection. The HDAg is characteristically positive in case of acute HDV infection. If the HDAg test is negative, it doesn’t exclude chronic HDV infection, and replication of HDV!
Demonstration of HBV/HDV-related chronic liver disease (CLD)
In HBV/HDV infected individuals, demonstration of CLD includes demonstration of chronic inflammation by elevated aminotransferases for >6 months (primarily GPT/ALT) and/or by histology (HAI>1) and/or demonstration of fibrosis (F≥1) by liver biopsy, and/or by transient elastography (FiroScan, liver stiffness ≥6.0 kPa), and/or by any other elastography or validated non-invasive methods (e.g.
FibroTest, ELF test, FIB-4-test). If the GPT/ALT is consistently elevated for >6 months and the titer of HBV DNA is above 2,000 IU/mL (2×103 IU/mL), liver biopsy as well as other fibrosis assessment methods can be omitted. Possible other causes of CLD must be considered and excluded.
Aminotransferases
Elevated GPT/ALT suggests active hepatitis but it may show permanently normal results in the immunotolerant phase of the infection, and it can also be intermittently normal in some HBeAg negative patients. Its long-term follow-up is essential for decision on therapy. Based on clinical studies, for patients with chronic hepatitis B the upper limit of normal GPT/ALT is 35 U/L for men and 25 U/L for women. Between these values and the (typically higher) normal values of a particular laboratory, results can be interpreted as ’borderline values’. Elevated GPT/ALT three occasions lasting at least for 6 months confirms CLD and supports therapy, as well as one elevated GPT/ALT before the start of the therapy if HBV/HDV infection certainly/presumably persists for more than 6 months.
Liver biopsy
Hepatitis activity index (HAI) > 1 with confirmed HBV infection for at least 6 months and/or fibrosis stage ≥1 on histology confirm CHB. In HBV/HDV infected patients liver biopsy might be relevant and recommended for differential diagnosis and demonstration of other liver diseases.
Non-invasive fibrosis assessment. Liver stiffness (LS) ≥6.0 kPa on transient elastography (FibroScan) and/or a fibrosis stage ≥1 with any other validated non-invasive fibrosis assessment method (e.g. other elastographies, FibroTest, ELF test, FIB-4-test) also confirm CLD (3, 4, 6, 7).
Treatment initiation
Indications of HBV/HDV therapy
Goals of HBV therapy
The primary aim of the therapy is the seroconversion of HBsAg to anti-HBs. If it is not achieved, the alternative aim is to reduce (or cease) the replication of HBV. Further, in case of HBeAg positivity, the aim is the seroconversion of HBeAg to anti-HBe. In this way, we can abolish/limit the inflammation in the liver, prevent or slow down progression, development of cirrhosis and liver failure, and the risk of HCC (8, 9, 10).
No cirrhosis
Treatment of non-cirrhotic HBV infected patients are recommended – independently of the HBeAg status –, if HBV-DNA is ≥2×103 IU/mL (2,000 IU/mL) and ALT is elevated. If ALT is normal, liver biopsy and/or non-invasive fibrosis assessment is necessary and antiviral therapy is indicated if HAI>1 and/or LS ≥6,0 kPa on transient elastography (FibroScan), and/or fibrosis stage ≥1 with any other validated non-invasive fibrosis assessment method (e.g. other elastographies, FibroTest, ELF test, FIB-4-test). If the titer of HBV DNA is <2×103 IU/mL, and no CLD can be demonstrated (GPT/ALT is normal, neither fibrosis nor active inflammation can be confirmed), therapy is not recommended (exceptions are mentioned in the section of special groups of patients) (5) – however these patients are to be followed for both viremia and liver disease activity.
Compensated cirrhosis
Any patients with cirrhosis must be treated if HBV DNA is detected – independently of the viral titer and activity. The following results suggest cirrhosis: bridging fibrosis or definitive cirrhosis (Knodell F3-F4 or Ishak F4-F5-F6) by histology, and/or by transient elastography (LS >9.6 kPa) and/or by any other validated non-invasive fibrosis assessment method (e.g. any other elastography, FibroTest, ELF test, FIB-4-test). Child-Pugh score <7 confirms compensated liver disease.
Decompensated cirrhosis
In case of HBsAg positivity, patients with decompensated cirrhosis must be treated independently of the titer of HBV DNA (even if the titer is negative)! Child-Pugh score ≥7 confirms decompensated liver disease.
HBV infection and chemotherapy, immunosuppressive or biological therapy
PCR for HBV DNA is recommended for patients with positive HBsAg and/or anti-HBc if they receive chemotherapy, immunosuppressive or biological therapy, or they’ve undergone organ/bone marrow transplantation. If the result is positive, the patient must be treated. Therapy might also be recommended for some patients with undetectable HBV DNA.
CHD
IFN/PEG-IFN therapy is recommended if anti-HDV IgM is positive – even if HBV DNA is not detectable! Patients with Child-Pugh A cirrhosis must be treated with PEG-IFN in case of HDV-Ag or anti-HD-IgM positivity – independently of activity and of HBV DNA detectability. If HBV DNA is also detectable, concomitant NA therapy can be considered. PEG-IFN is not recommended in patients with decompensated cirrhosis.
Additional assessments, measures prior to treatment
- General clinical assessment, in particular, signs of hepatic decompensation (Child-Pugh parameters), signs of co-morbidities.
- HBeAg, anti-HBe serologies.
- HBsAg titer is useful in patients with chronic HBV infection, primarily for those, to be treated with INF/PEG-INF. Recommended, but not mandatory.
- HAV serology. Vaccination against hepatitis A is recommended for susceptible patients, before or during the therapy.
- Anti-HCV, anti-HIV 1-2. If positive, appropriate diagnostics and therapy.
- Renal functions, TSH.
- Abdominal US (and further imaging if HCC is suspected).
Examinations for co-existing liver diseases: autoimmune, alcoholic, metabolic liver diseases (including NAFLD, NASH).
- Before starting therapy, the patient must be consulted by the attending/treating physician on HBV/HDV infections, on activity and stage of liver disease, on goals, modalities and conduct of therapy, on treatment options, on prognosis, on preventive measures, and on other relevant issues to obtain a signed informed consent of the patient.
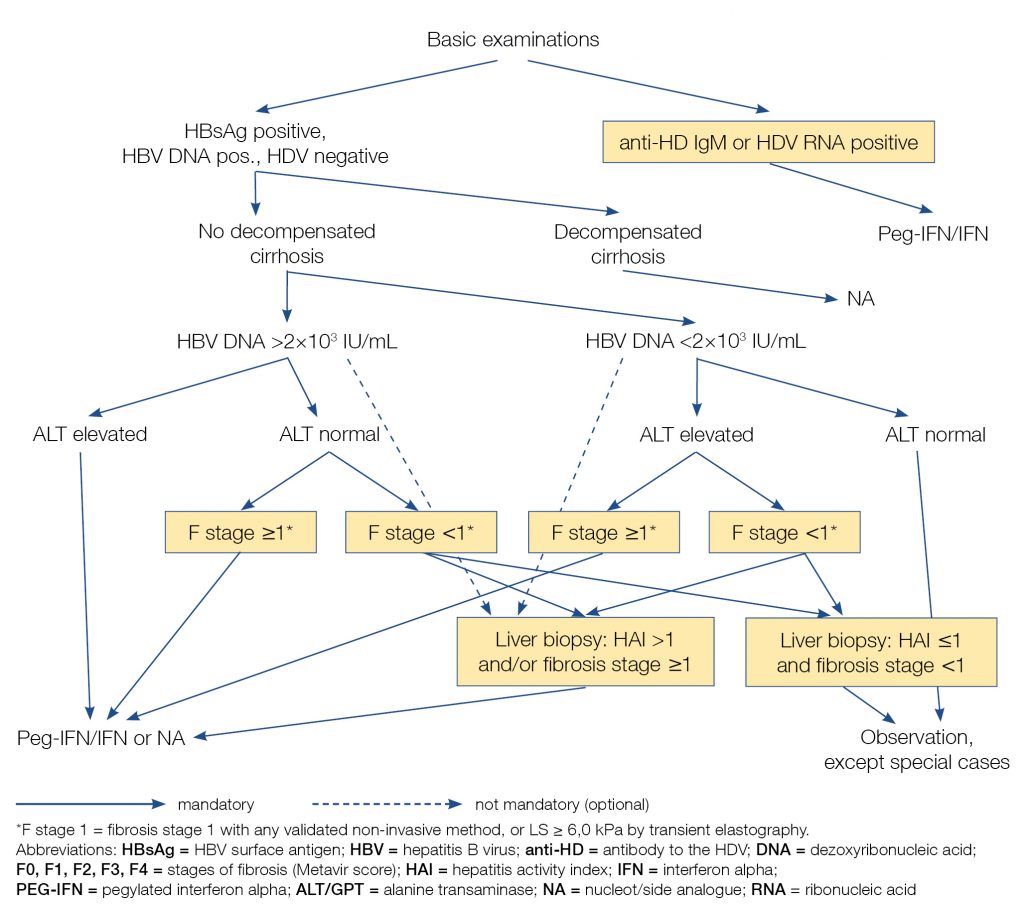
Figure 1 summarises algorithm of diagnosis and treatment of chronic hepatitis B with or without hepatitis D.
Figure 1. Algorithm of diagnosis and treatment of chronic hepatitis B with or without hepatitis D
Treatment options
In most cases, the first choice of therapy can be either pegylated interferon-alpha (PEG-IFN) or a NA. For treatment decision, patient’s preference is to be taken into account.
Pegylated interferon alpha (PEG-IFN) therapy
First line PEG-IFN therapy is recommended (if no contraindication):
-
- In HDV co-infected patients (anti-HD IgM, HDV RNA, or HDAg positive). Concomitant NA therapy for CHB might be considered.
Amongst other options, PEG-IFN therapy is preferred (but not mandatory):
- HBV therapy naive young patients (threefold of the upper limit of normal,
- HBV DNS <2×108 IU/mL,
- HAI ≥2,
Dose of PEG-IFN-alpha-2a: 1×180 mg per week, sc; 1×135 mg per week, sc if creatinine clearance is less than 30 mL/min.
Duration: 48 weeks; 72 weeks for anti-HD-IgM positive patients.
PEG-IFN is contraindicated in patients with decompensated cirrhosis.
Measuring titer of HBV DNA and serologies during the IFN/PEG-IFN therapy
HBV DNA is recommended on week 24 of the therapy, at the end of therapy, on week 24 and week 48 after the therapy. In case of HBeAg positivity, HBeAg and/or anti-HBe monitoring must be performed with the same schedule. In addition, monitoring of HBsAg titer is also recommended if available. After achieving seroconversion of HBeAg to anti-HBe, HBsAg test is recommended in every year if HBV DNA cannot be detected. When HBsAg becomes negative, anti-HBs serology must be performed.
Evaluation of success of PEG-IFN therapy
- On-treatment viral response: HBV DNA <2×103 IU/mL at week 24 of the therapy
- Permanent viral response: HBV DNA <2×103IU/mL at the end of the therapy, and at week 24 and 48 after therapy.
- Non-adequate on-treatment viral response: not properly defined. Switching to NA therapy may be considered if the titer of HBV DNA ≥2×103IU/mL and/or the HBV DNA load reduction is <1 log10 at week 24 of therapy.
- Breakthrough: re-occurrence of HBV viraemia or 1 log10 elevation of the HBV DNS load any time during therapy. Switching to NA therapy is recommended.
- Multiple studies have confirmed that reduction of the titre of HBsAg during the PEG-IFN is a positive predictor of permanent viral response and also of HBsAg seroconversion.
Additional monitoring during IFN/PEG-IFN therapy
- Laboratory monitoring is necessary during the IFN/PEG-IFN therapy: full blood count, GPT/ALT, GOT/AST, serum bilirubin (in every 4 weeks), serum creatinine, blood sugar, TSH, and uric acid (in every 12 weeks).
Additional monitoring after IFN/PEG-IFN therapy
- During (and after) the therapy, abdominal US must be performed in annually; every 6 months for patients with cirrhosis.
- After the end of the therapy, liver function and full blood count must be monitored at least every 6 months.
- For HBsAg positive patients and/or if HBV DNA is detectable, HBV DNA must be followed annually after the end of therapy. If HBV DNA is undetectable, monitoring of HBsAg is also recommended. If HBsAg becomes negative, anti-HBs serology must be performed.
Nucleotide/nucleoside analogue (NA) therapies
If not contraindicated, NA therapy can be chosen for the following patients:
- Amongst other options, a NA can be the first choice of therapy in any anti-HD IgM and HDV RNA negative HBV infected patients, regardless of HBeAg status.
- If no-adequate on-treatment viral response to IFN therapy.
- If there is breakthrough during IFN therapy.
- If there is no response after the 48-week IFN therapy.
- If after response to the IFN therapy, there is a relapse.
- As a second choice of therapy in case of drug resistance (in such a case, it can be administered even in combination).
- For HBsAg and/or HBV DNA positive patients before, during and after biological-, immunosuppressive-, or chemotherapy.
- HBsAg and/or HBV DNA positive patients who have undergone organ transplantation.
- Prophylaxis for patients with HBV who undergone organ or bone marrow transplantation
- In any stage of cirrhosis caused by HBV.
- In case of any contraindications of IFN.
- Even for anti-HDV IgM positive patients if the titer of HBV DNA is consistently or intermittently >2000 IU/mL and IFN therapy is contraindicated or it is not effective.
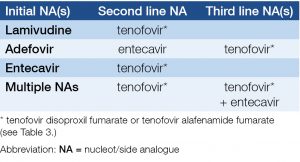
- Recommendations for unsuccessful NA therapy or NA resistance are listed in Table 3.
Table 3. Recommendations for selection of entecavir (ETC) or tenofovir alafenamide fumarate (TAF) therapy instead of tenofovir dipivoxil fumarate (TDF)*
Duration of NA therapy
- NA therapy can be terminated if there is no detectable HBV DNA and HBsAg becomes negative (independently of anti-HBs!).
- In case of HBsAg positivity, terminating the NA therapy can be considered if ALT/GPT <ULN, there is no cirrhosis, progression of fibrosis cannot be detected, regular follow up of the patient after the cessation of therapy can be guaranteed, and
- for HBeAg positive patients: HBeAg to anti-HBe seroconversion is achieved and HBV DNA cannot be detected for 12 months of maintenance therapy,
- for HBeAg negative patients: if fibrosis stage is <3, and HBV DNA is not detectable for at least 3 years, and there is no (and there wasn’t) any extrahepatic manifestations of HBV.
HBV DNA and serology follow up during NA therapy
- HBV DNA test is recommended in every 6 months (every three months in cirrhotics) after the initiation of NA therapy, and whenever increase of GPT/ALT or other clinical/laboratory parameter suggests potentially inadequate viral response or breakthrough during the therapy.
- After the first year of ETC, TDF or TAF therapy, annual HBV DNA testing is sufficient, if last HBV DNA is not detectable and GPT/ALT is normal.
- For HBe positive patients on NA therapy, HBeAg and/or anti-HBe testing is recommended in every six months.
- For HBeAg negative patients on NA therapy, or after HBeAg to anti-HBe seroconversion, annual HBsAg testing is recommended if HBV DNA is undetectable. After HBsAg becomes negative, an anti-HBs serology must be performed.
Additional follow up during NA therapy
- During NA therapy, liver functions and blood counts to be followed in every 3 months. Significant increase in GPT/ALT suggests HBV breakthrough due to a NA resistance-associated substitution/mutation (RAS). RAS testing is recommended – if available. If RAS is confirmed, or RAS testing is not available, switching to another medication is recommended.
- During TDF/TAF therapy renal functions to be followed every month in the first three months of therapy, thereafter – if stable – at least in every three months.
- During (and after) HBV therapy, annual abdominal US is necessary to screen for HCC. Patients with cirrhosis are to be examined in every 6 months.
Evaluation of on-treatment and post-treatment efficacy of NA therapy
- Complete viral response: HBV DNA is not detectable.
- Partial viral response: There is a reduction of HBV DNA titer at month 6 of therapy or later on, to >2×103 IU/mL, but HBV DNA is still detectable. Except the patient receives ETC, TDF or TAF, switching to another NA or a combined NA therapy is recommended, or IFN therapy can also be considered. In case of switch to INF therapy, ongoing ETC, TDF or TAF therapy to be continued.
- Breakthrough: Re-occurrence of viraemia or increase of HBV DNA titer by 1 log10. RAS testing is recommended – if available. If RAS is confirmed, or RAS testing is not available, switching to another medication is recommended.
- Permanent viral response: titer of HBV DNA <2.000 IU/mL during and at least 1 year after the end of NA therapy.
Recommendations at the end and after NA therapy
- At the end of therapy, and thereafter at least every 6 months (more frequently if unstable): full blood counts, liver functions.
- At the end of therapy, and 6 and 12 months thereafter: HBV DNA; for HBeAg positive patients, HBeAg and anti-HBe. For HBeAg negative patients, HBsAg and anti-HBs.
- After 48 weeks of post-treatment follow-up: HBV DNA annually in the case of HBsAg positivity and/or if HBV DNA is detectable. In case of undetectable HBV DNA, HBsAg testing is recommended. If HBsAg becomes negative, anti-HBs testing is recommended.
- After discontinuation of HBV therapy: annual abdominal US to screen for HCC; every 6 months in patients with cirrhosis are to be examined in.
Entecavir (ETC)
- ETC is one of the most effective types of NAs and there is only a very little risk of drug resistance. According to the European guideline, it is one of the first drugs of choice of NA therapy. Its effectiveness and safety are confirmed even for patients with decompensated cirrhosis of the liver.
- ETC is the first choice of NA therapy for patients with renal impairment – dose to be adjusted according to the label.
- Because of the risk of developing another resistance, in case of LAM resistance, ETC is recommended only if TDF/TAF are not available or contraindicated.
- In case of resistance to ETC or if there is detectable HBV DNA at week 96 of ETC therapy, switching to TDF or TAF is recommended.
- Dose of ETC: For NA näive patients: 1×0.5 mg daily, per os.; 1×1,0 mg daily, per os in case of resistance to LAM (not recommended if TDF/TAF can be prescribed) and in patients with decompensated cirrhosis. For patients with decompensated cirrhosis, TDF or TAF might be more cost-effective than ETC, thus TDF/TAF is recommended (if there is no contraindication). Reduced dose might be appropriate for patients with renal impairment, according to the label.
Tenofovir disoproxil fumarate (TDF)
- TDF is one of the most effective NAs, resistance is not known so far. Amongst other options, the European HBV guideline recommends it as one of the first drugs of choice of NA therapy.
- TDF is recommended in case of drug resistance to LAM, ADV, or ETC.
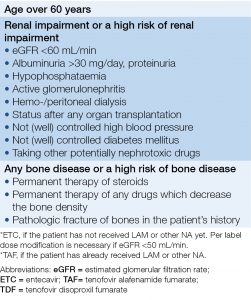
- However, ETC or TAF is recommended instead of TDF for patients with bone or kidney diseases or if there is a high risk of these diseases (Table 4).
- Dose of TDF: 1×245 mg daily, per os.
- During the therapy, kidney function must be followed regularly (every month during the first three months, then at least in every 3 months if stable).
Table 4. Recommendations for second and third line therapies if NA resistance is confirmed or suspected
Tenofovir alafenamide fumarate (TAF)
- TAF is an alternative of, and as effective as TDF. According to the clinical studies, TAF affects kidney function and bone density in a lower extent than TDF.
- ETC or TAF is recommended instead of TDF for patients with bone or kidney diseases or if there is a high risk of these diseases (Table 4).
- In Child-Pugh B and C stages of cirrhosis, we have no data about the effectiveness and safety of TAF, thus it is not recommended. In every other case, indications and duration of the therapy are the same as of TDF. After it was accepted, – amongst other options – it could be recommended as a first-choice therapy (depending on the price).
- Dose of TAF: 1×25 mg daily, per os.
Adefovir dipivoxil (ADV)
- Currently not available in Hungary.
- ADF is less effective than TDF, and the risk of resistance is higher. For this reason, ADF is not recommended as first line therapy, if other options are available (particularly not as monotherapy).
- Upon TDF and TAF are contraindicated or not available, ADV+LAM combination therapy is recommended in case of LAM resistance. However, switching to TDF or TAF is more effective in case of LAM resistant HBV.
- In case of resistance to ADV, switching to ETC or TDF or TAF is recommended. If the viral titer is ≥108 IU/mL, ETC is recommended.
- Dose of ADV: 1×10 mg daily, per os. For patients with renal impairment, the dose to be reduced according to the label.
Lamivudine (LAM)
- The risk of drug resistance during LAM therapy is high, especially if it is administered as monotherapy. Consequently, initiating new LAM therapy for CHB is indicated only if other NAs contraindicated or not available.
- Ongoing LAM therapy can be continued as long as there is no advanced liver disease (F<3), and HBV DNA is <20 IU/mL.
- Preventive LAM therapy can also be considered during and after biological-, immunosuppressive-, or chemotherapy for HBV DNA and HBsAg negative, anti-HBc positive patients with no advanced liver disease (F<3) if expected duration on LAM therapy is not longer than 1 year
- For patients with cirrhosis, LAM monotherapy is contraindicated.
- HBV DNA titer ≥20 IU/mL during LAM therapy suggests LAM resistance. Switching to TDF or TAF, or – if TDF or TAF is contraindicated or not available – combination of LAM with ADV is recommended. ETC therapy carries a higher risk of resistance in case of LAM resistance.
- Dose of LAM: 1×100 mg daily, per os. For patients with renal impairment, dose reduction or switching to ETC (monotherapy) is recommended, depending on the eGFR.
NA plus PEG-IFN add-on therapy (11)
- Add-on NA+PEG-IFN therapy can be chosen for HBeAg positive or HBeAg negative patients with CHB if HBeAg or HBsAg seroconversion was not achieved after at least one-year of NA monotherapy, respectively.
- Dose: same as for monotherpies.
- Recommended duration: 48 weeks. Thereafter, continuation of NA therapy is recommended as long as for NA monotherapy.
- Lab tests during and after the combined therapy: same as for PEG-IFN and NA monotherapies.
Special patient groups
HBeAg positive patients above 30 years
Antiviral therapy is recommended for HBeAg positive patients if they are older than 30 years and the titer of HBV DNA is high (>2×103 IU/mL) even if the value of the GPT/ALT is normal. For this group, antiviral therapy (IFN/PEG-IFN, ETC, TDF or TAF) is recommended independently of the degree of activity and fibrosis.
Patients with increased complication risk based on family history
Regardless of HBeAg status, patients with chronic HBV infection and family history of HCC or of cirrhosis and/or extrahepatic manifestation, antiviral therapy should be considered (IFN/PEG-IFN, ETC, TDF or TAF).
Patients with compensated cirrhosis
For patients with compensated cirrhosis, std-IFN, PEG-IFN alpha-2a, ETC, TDF or TAF are recommended because of the low risk of drug resistance. NA therapy must be continued lifelong. Discontinuation of NA therapy may be considered one year after HBeAg to anti-HBe, or – with more confidence – after HBsAg to anti-HBs seroconversion, if HBV DNA is undetectable.
Patients with decompensated cirrhosis
HBsAg positive patients with decompensated cirrhosis require lifelong antiviral therapy (independently of
detectability or titer of HBV DNA). ETC must be administered in a high dose (1.0 mg per day) while TDF must be administered in a normal dose (1×1.25 mg per day). If there is no contraindication, TDF is the first choice NA therapy or switching to TDF is indicated (based on cost-effectiveness). TAF might be a better option, especially in cases with impaired kidney function, however the safety and efficacy of TAF has not been confirmed in these patients so far, thus it is not recommended. Discontinuation of NA therapy may be considered one year after HBeAg to anti-HBe, or – with more confidence – after HBsAg to anti-HBs seroconversion, if HBV DNA is undetectable.
HBV infected liver transplant recipients
Before the transplantation, each HBsAg positive patient is to receive NA therapy (ETC or TDF) in order to reach the lowest possible titer or undetectability of HBV DNA by the time of transplantation.
After the transplantation, NA therapy seems to be effective and safe, and recommended lifelong to prevent recurrence of HBV. HBsAg positive patients at the time of the transplantation are also to receive HBIG therapy.
Patients after liver transplantation from HBsAg negative/anti-HBc positive donor
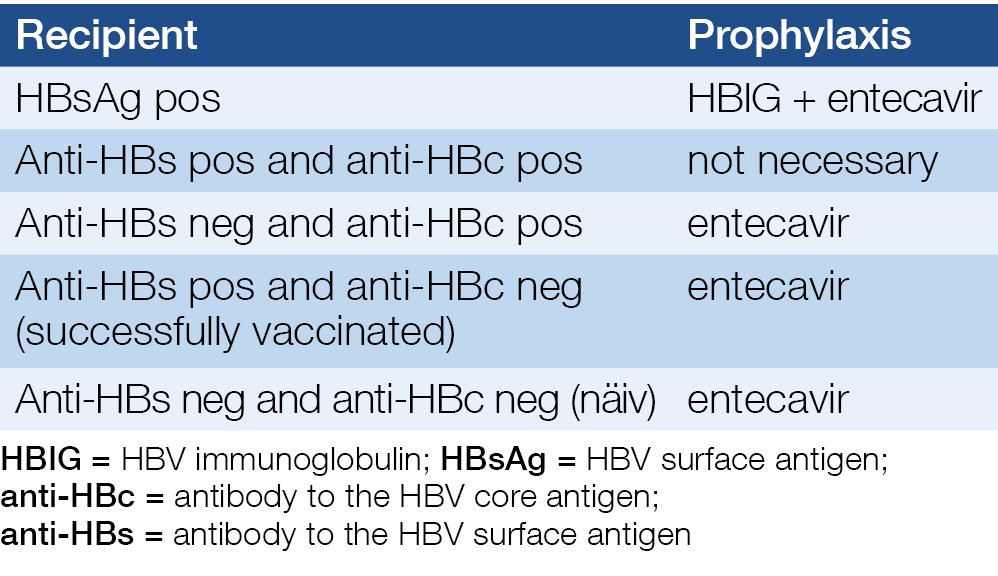
Recommended prophylaxis after the transplantation is listed in Table 5.
Solid organ graft recipients transplanted from a HBsAg negative/anti-HBc positive donor
Transplantation of a HBsAg negative/anti-HBc positive donor organ to a HBsAg negative/anti-HBc positive or negative recipient is not contraindicated if the anti-HBs titer ≥10 IU/mL at both the donor and the recipient. The recipient must be on ETC or TDF therapy for at least 6 months after transplantation. ETC must be preferred in case of renal impairment or if there is a high risk of it. Regular HBV serologies (3 and 6 months after transplantation, every 6 months thereafter) and HBV DNA PCRs (6 months after transplantation, thereafter annually) are recommended. If there is any suspicion of viral reactivation, unscheduled HBV DNA testing is recommended.
Table 5. Recommended prophylaxis after the transplantation
Patients with solid organ transplantation from HBsAg positive donor
Transplantation of a solid organ from a HBsAg positive donor is generally not recommended, but can be acceptable in desperate circumstances. In particular, liver transplantation can only be acceptable if there is no relevant liver damage in the donor liver by histology.
After the transplantation of any organs, NA prophylaxis is mandatory. Administration of HBIG is also necessitated if the anti-HBs titer in the recipient is <100 mIU/L. Follow up of HBV DNA, HBsAg and anti-HBs is recommended in every three months in the first year; thereafter in every 3-6 month.
Patients with HBV and HIV co-infections
Indication and conduct of HBV therapy is the same as for HIV negative patients. In most cases, simultaneous de novo therapy of HIV and HBV is recommended (TDF, emtricitabine, and a third anti-HIV medication)
If HBV therapy is initiated prior to HIV therapy, ADV or telbivudine should be chosen because they have no confirmed effect on HIV virus. LAM, ETC or TDF monotherapy for HBV is contraindicated without concomitant therapy for HIV in co-infected patients, because as monotherapy they may induce HIV viral resistance due to their anti-HIV activity.
Patients with HBV and HCV co-infections
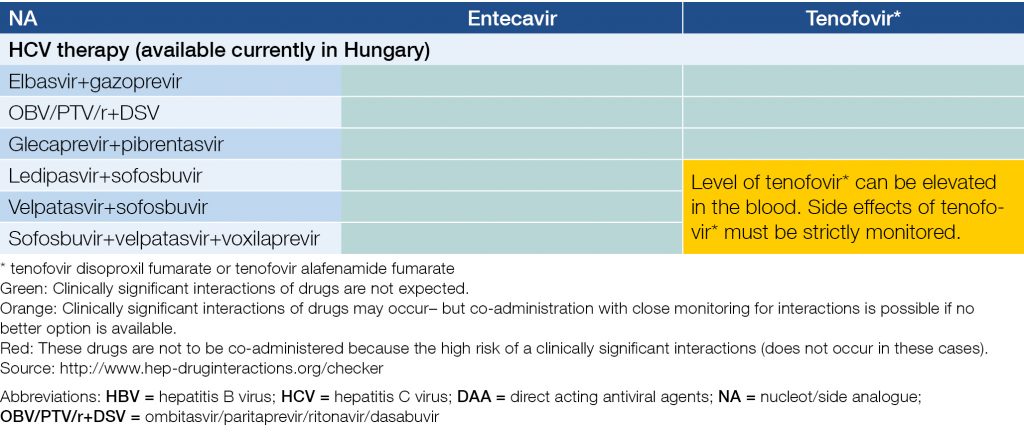
The titer of HBV DNA is usually low or undetectable in HCV co-infected patients. HCV co-infected patients can be treated for HBV with NAs, according to the guidelines for HBV mono-infection. Furthermore, reactivation of HBV may occur during or after the DAA therapy of HCV; if it occurs, NA therapy is to be initiated. HCV can also be treated in HBV co-infected patients according to the guidelines for HCV mono-infection. Efficacy is the same as for HCV mono-infected patients. HBsAg, anti-HBs and anti-HBc serologies are necessary before DAA therapy. HBsAg positive patients should receive NA therapy at the same time, because of the HBV infection. This NA therapy must be continued at least 24 weeks after the end of the therapy of HCV. HBsAg negative but anti-HBc positive patients require strict follow up during HCV therapy: ALT/GPT in every month during the therapy and 1, 3, and 6 months thereafter. If the HCV RNA is negative but ALT/GPT is not normalized or rises during or after HCV therapy, HBsAg and HBV DNA testing are recommended. Co-administration of NA therapy is recommended if HBV flare is confirmed or suspected! Drug-drug interactions of may occur during simultaneous therapy of HBV and HCV (Table 6).
Table 6. Potential interactions of recommended NAs and DAAs during concomitant treatment of HBV and HCV
Patients with chronic hepatitis D co-infection
IFN/PEG-IFN is the only evidence-based therapy for HDV infection. Duration of the therapy is one and a half year; in case of a relapse, the therapy should be repeated. NAs don’t have any effect on the replication of the HDV but co-administration of a NA can be recommended if HBV DNA is >2×103 IU/mL consistently or intermittently or other indications of HBV treatment mandate.
After a successful PEG-IFN therapy of chronic hepatitis D (IgM anti-HD and/or HDV RNA negative), therapy must be continued as for chronic HBV infection.
Patients with fulminant or progressing severe acute hepatitis B
NA therapy may be beneficial. Some publications report benefit of LAM therapy, but this is not properly proved in randomized clinical trials yet. ETC, TDF, TAF have excellent anti-HBV potential and low risk of viral resistance should be preferred. Optimal duration of the therapy is unknown, but continuation as long as in case of CHB is reasonable and recommended.
Differentiation of real severe acute hepatitis B and from a relapse of CHB can be difficult. Liver biopsy might be necessary. In both cases, NA is the therapy.
HBV infected children
The effectiveness and safety of IFN, LAM, ADV, and entecavir are confirmed for children above 2 years of age.
HBV infected healthcare workers (HCWs)
For HBsAg and HBV DNA positive HCWs, IFN/PEG-IFN, ETC, TDF or TAF is recommended.
HBV infected women who plan pregnancy
No advanced fibrosis (F≤2)
Anti-HBV therapy with a 48 week IFN/PEG-IFN prior to pregnancy may be considered with a 6 months post-treatment interval before pregnancy. Alternatively, therapy of HBV should be postponed after delivery. Proper contraception is mandatory during IFN/PEG-IFN therapy.
Compensated, but advanced liver disease (F3 or F4 fibrosis)
A 48 week IFN/PEG-IFN therapy with a 6 months post-treatment interval before pregnancy is recommended for HBV infected women with advanced fibrosis but compensated liver disease. Proper contraception is mandatory during IFN/PEG-IFN therapy. If IFN/PEG-IFN therapy is ineffective or contraindicated, tenofovir therapy to be initiated and to be continued during and after the pregnancy (as long as for CHB treatment), after detailed counseling with the patient (and family) on potential risks and benefits of this therapy during pregnancy (15).
Decompensated liver disease
Childbearing potential is low, pregnancy carries high maternal and fetal risks, INF/PEG-IFN is contraindicated, NAs are either contraindicated or not recommended in first two trimesters of pregnancy. For this reasons, pregnancy is not recommended for HBV infected women with decompensated liver disease. TDF (or ETC) therapy is to be initiated, and liver transplantation is to be considered.
HBV infected pregnant women (12, 13)
According to the FDA, LAM, ADV, and ETC classified into pregnancy risk category C, telbivudine and TDF into category B. IFN/PEG-IFN is contraindicated.
Recommendations for initiating new HBV therapy are identical as for HBV infected women who plan pregnancy.
Continuation or discontinuation of an ongoing HBV therapy is to be re-considered and to be counseled with a woman who becomes unexpectedly pregnant. TDF therapy may be continued, while IFN/PEG-IFN, LAM, ADV, or ETC therapy should be switched to TDF (mandatory in fibrosis stage F3 or F4).
To prevent perinatal transmission, in all pregnant women with HBV DNA >2×105 IU/ml or HBsAg >1×104 IU/ml, TDF prophylaxis should start at week 24–28 of gestation and continue for up to 12 weeks after delivery.
HBsAg positive mothers are to be strictly followed after delivery, because of the risk of acute flare of HBV hepatitis (even if the HBV infection did not require treatment previously).
Newborns of HBV infected mother
Passive and active immunization of newborn of a HBV infected mother (HBsAg and/or HBV DNA positive) is mandatory in the perinatal period (within 12 hours after birth). However, even with passive and active immunization, the risk of the vertical transmission remains still high (>10%) if viral load of mother is high in the prenatal period. To decreases the viral load and make active plus passive immunization more effective, from week 24-28 of gestation TDF therapy is recommended for pregnant women with HBV DNA >2×105 IU/ml or HBsAg >1×104 IU/ml (13).
Breast feeding is NOT contraindicated, neither for HBsAg positive untreated women nor during TDF therapy! Concentration of TDF in breast milk is relatively low.
Patients who receive chemotherapy, immunosuppressive or biological therapy, bone marrow or stem cell transplantation (together: interventions)
For patients with either inactive HBV infection (HBsAg positive, no liver damage, no sign of hepatitis) or occult HBV infection (HBsAg negative, anti-HBc positive, no liver damage, no sign of hepatitis) receiving any of such therapies carries a severe risk of reactivation of HBV infection, including fulminant hepatitis. Therefore, HBsAg, anti-HBs and anti-HBc are to be screened prior to any of these interventions. If any of the results is positive, hepatology consultation is recommended. Furthermore, non-immune (seronegative) patients should be actively immunized against hepatitis B.
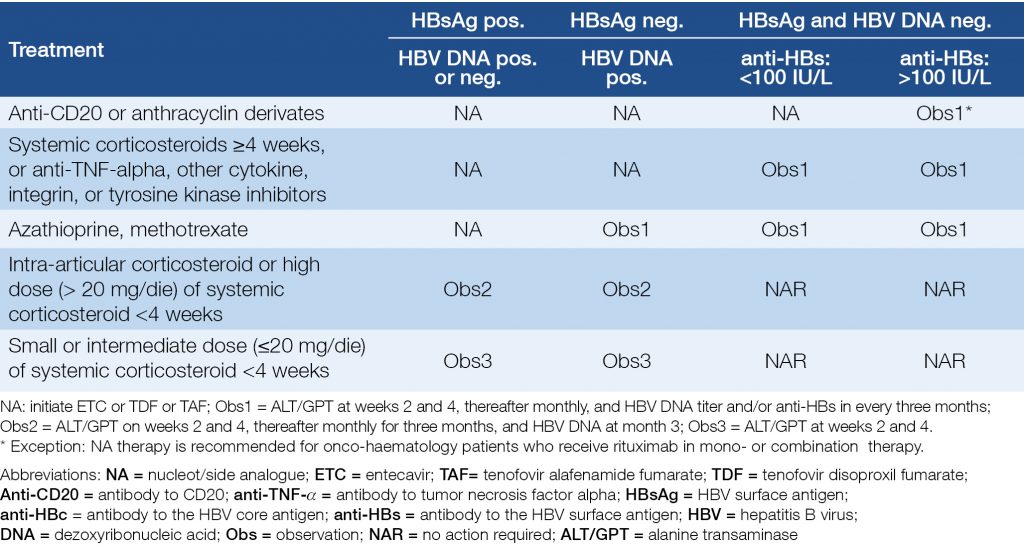
Regardless of HBsAg status, PCR for HBV DNA is recommended for all anti-HBc positive patients. (14) For HBsAg and/or HBV DNA positive patients, NA therapy (ETC, TDF or TAF) is to be initiated prior to (preferably) or during such interventions, and to be continued for at least one year after the completion of intervention. In case of rituximab mono- or combination therapy, NA prophylaxis should continue for at least one and a half years after stopping the intervention, and close monitoring should continue for an additional year threreafter. Anti-HBc positive but HBsAg and HBV DNA negative patients are to be strictly followed during and after such interventions: monthly GPT/ALT, and anti-HBs titer every 3 months (if previous anti-HBs titer is above 100 IU/L) or HBV DNA level every 3 months if anti-HBs is below 100 IU/L.
If regular follow up of HBV DNA is not available or the anti-HBs titer is below 100 IU/L, NA prophylaxis is recommended for all anti-HBc positive, HBsAg negative patients who receive rituximab and/or combination chemotherapy because of a malignant hematologic disease (15, 16, 17).
NA prophylaxis is also recommended for anti-HBc positive, HBsAg and HBV DNA negative patients who receive bone marrow or stem cell transplantation (independently of the titer of anti-HBs) (18).
ETC, TDF or TAF is recommended for prophylaxis; LAM can only be used if its duration is not longer than one year. Duration of prophylactic NA therapy is be consulted between the haematologist(s) and hepatologist(s).
In case of HBV reactivation, NA therapy is to be initiated, and to be continued for at least one and a half years after completion of intervention.
Table 7 and Table 8 show the risks and recommendations for patients who receive immunosuppressive or biological therapy (19, 20, 21).
Table 7. Risks of reactivation of HBV in case of various immunosuppressive and biological therapies
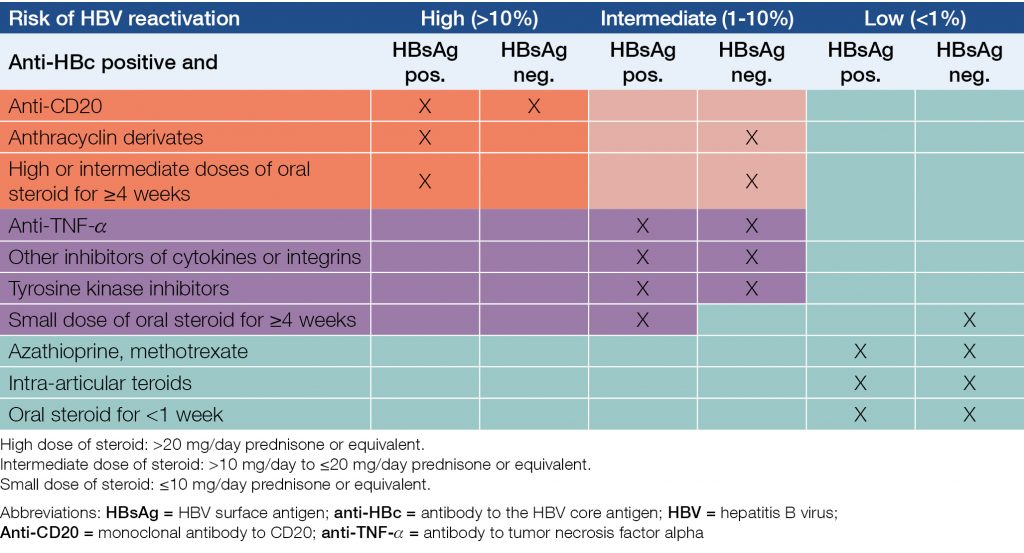
Table 8. Recommendations for anti-HBc positive patients who receive biological or immunosuppressive therapy
Patients on hemodialysis or after kidney transplantation
Either a NA or IFN/PEG-IFN therapy can be chosen for patients with chronic kidney disease. ETC is the optimal therapy for kidney transplanted patients – IFN/PEG-IFN is contraindicated.
Patients with extrahepatic manifestations
IFN, PEG-IFN or a NA can be considered for HBsAg positive and/or HBV DNA positive patients with HBV-related extrahepatic manifestations. However, IFN/PEG-IFN may be even unfavorable, harmful, or contraindicated in certain immune-mediated extrahepatic manifestations.
In special cases, NA therapy combined with plasmapheresis may increase effectiveness.
Conclusions
HBV infection can cause severe (even fatal) acute hepatitis, and may lead to end stage liver disease as well as to HCC (even in patients with otherwise not relevant liver disease). These outcomes and related financial burden can be greatly reduced with immunization and screening programs, and with effective therapies.
The incidence, and – in a smaller extent – the prevalence of HBV have been recently reduced in countries with immunization programs, including Hungary. Unfortunately, prevalence is still significant in non-immunized individuals (born before 1986 in Hungary). Extension of immunization program to yet not included populations – especially for those at risk – is at upmost importance, and may prove cost-effective from health-economy and societal aspects.
As of therapy, it is considered effective if replication of HBV is blocked completely, or reduced to a minimal level. If it is achieved, risk of complications is low, work-ability and life-expectancy are close to non-infected individuals. Effective therapies are basically available in Hungary. However, complete “cure” of HBV with registered therapies is nowadays not possible.
Early recognition and per guideline treatment of HBV infection require organized high-scale screening programs. Without such programs, reduction of HBV incidence and related mortality goal set by WHO cannot be achieved in Hungary by 2030.
Acknowledgments
We express our gratitude to Árpád Kiss (Satco Ltd) for his technical assistance in the preparation of this paper.
2. Hungarian Ministry of Human Resources. Management of chronic B and D hepatitis. Protocol for of the Hungarian Ministry of Human Resources (A Nemzeti Erőforrás Minisztérium Szakmai Protokollja a B- és D-hepatitis kezeléséről). Egészségügyi Közlöny 2011; 61(7): 1379–1385. www.kozlonyok.hu/kozlonyok/Kozlonyok/6/PDF/2011/7.pdf (Hungarian) https://doi.org/10.1556/650.2017.30689
3. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. European Association for the Study of the Liver. J Hepatol 2017; 67: 370–398. https://doi.org/10.1016/j.jhep.2017.03.021.
4. Terrault NA, Lok SF, McMahon J, et al. Update on prevention, diagnosis and treatment of chronic hepatitis B AASLD 2018 Hepatitis B Guidance. Hepatology 2018; 67: 1560–1599. https://doi.org/10.1002/hep.29800.
5. Horváth G. New drugs for the treatment of chronic hepatitis B and interdisciplinary aspects of chronic hepatitis B virus infection (A hepatitis B vírusfertőzés új gyógyszerei és interdiszciplináris vonatkozásai). Orv Hetil 2013; 154: 1142–1150. (Hungarian) https://doi.org/10.1556/OH.2013.29625
6. Pár A, Pár G. Non-invasive fibrosis assessment in chronic hepatitis C: aspartate-aminotransferase to platelet ratio index (APRI) and transient elastography (FibroScan). (Nem invaziv fibrosisdiagnosztika kronikus C-hepatitisben: aszpartat-aminotranszferaz/ thrombocyta hanyadosindex (APRI) es tranziens elasztografia (FibroScan)). Orv Hetil 2010; 151: 1951–1955. (Hungarian) https://doi.org/10.1556/OH.2010.28978
7. Horváth G. New non-invasive tool for assessment of liver fibrosis: transient elastography. (A majfibrosis meghatarozasanak uj, noninvaziv modszere: tranziens elasztografia) (FibroScan). Orv Hetil 2011; 152: 860–865. (Hungarian) https://doi.org/10.1556/OH.2011.29094
8. Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295: 65–73. https://doi.org/10.1001/jama.295.1.65
9. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381: 468–475. https://doi.org/10.1016/S0140-6736(12)61425-1.
10. Shim JH. Efficacy of entecavir in teratment-naive patients with hepatitis B virus-related decompensated cirrhosis. J. Hepatol 2010; 52: 176–182. https://doi.org/10.1016/j.jhep.2009.11.007.
11. Brouwer WP, Xie Q, Sonneveld MJ, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen positive chronic hepatitis B. A multicenter randomized trial (ARES study). Hepatology 2015; 61: 1512–1522. https://doi.org/10.1002/hep.27586.
12. Bzowej NH. Hepatitis B therapy in pregnancy. Curr Hepat Rep 2010; 9: 197–204. https://doi.org/10.1007/s11901-010-0059-x
13. Han GR, Cao MK, Zhao W, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. Br J Hepatol 2011; 55: 1215–1221. https://doi.org/10.1016/j.jhep.2011.02.032.
14. Raimondo G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection. J Hepatol 2007; 46: 160–170. https://doi.org/10.1016/j.jhep.2006.10.007
15. Evens AM, Jovanovic BD, Su YC, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol 2011; 22: 1170–1180. https://doi.org/10.1093/annonc/mdq583.
16. Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol 2007; 136: 699–712. https://doi.org/10.1111/j.1365-2141.2006.06465.x
17. Marzano A, Angelucci E, Andreone P, et al. Prophylaxis and treatment of hepatitis B in immunocompromised patients. Dig Liver Dis 2007; 39: 397–408. https://doi.org/10.1016/j.dld.2006.12.017
18. Vigano M, Vener C, Lampertico P, et al. Risk of hepatitis B surface antigen seroreversion after allogeneic hematopoietic SCT. Bone Marrow Transplant 2011; 46: 125–131. https://doi.org/10.1038/bmt.2010.70
19. Lok ASF, Bonis PAL. Hepatitis B virus reactivation associated with immunosuppressive therapy. Available: http://www.uptodate.com/contents/hepatitis-b-virus-reactivation-associated-with-immunosuppressive-therapy.
20. Reddy KR, Beavers KL, Hammond SP, et al. New Guidelines for Managing Hepatitis B Reactivation During Immunosuppressive Therapy. Gastroenterology 2015; 148: 215–219. (available: http://www.medscape.com/viewarticle/843497).
21. Perillo PR, Gish R, Falk-Ytter YT. American Gastroenterological Association Institute Technical Review on Prevention and Treatment of Hepatitis B Reactivation During Immunosuppressive Drug Therapy. Gastroenterology 2015; 148: 221–244. (available: http://www.gastrojournal.org/article/S0016-5085(14)01330-4/abstract) https://doi.org/10.1053/j.gastro.2014.10.038
