Esetismertetés / Case report
Lipoma of the coecum with secondary changes of the surface
Summary
Our endoscopically and histopathologically well documented case report is about a lipoma of the colon, which is a relatively rare benign mesenchymal tumor found in the large intestine. The lipoma was characterized as a pedunculated polyp in the cecum, intermittently translocating into terminal ileum and obstructing Bauhin's valve, resulting in clinical symptoms. During patient examination, lipomas present with differential diagnosis challenges due to their close resemble to other disorders, which might occur much more commonly are more probable to be malignant. Furthermore, bigger lipomas can also harbour secondary changes, such as erosion, ulceration, bleeding and necrosis. Although endoscopic and imaging techniques offer assistance in characterizing lipomas, the final diagnosis still relies heavily on the resecting and histologic examination of the entire polypoid/tumor-like outgrowth of the mucosa. Superficial sampling might also lead to misdiagnosis. Our case had inflammatory, erosive and also hyperplastic surface which could have been concluded as a hyperplastic polyp of colon, a much more common occurrence.
Core tip
Our case report describes a lipoma, a benign mesenchymal tumor of the colon, which occurs quite rarely, but can mimic more frequently found and possibly malignant polyps. Therefore, differential diagnosis is a crucial importance. The bigger the lipoma is, the higher chance it will harbour secondary changes (inflammation, ulceration, necrosis, hyperplasia), leading to further differential diagnostic complications, especially with superficial sampling. Our pedunculated lipoma had demonstrated hyperplastic/inflammatory changes due to its mobility on the long stalk, emphasizing the importance of a complete resection and histological examination of the entire lesion in order to arrive at the final diagnosis.
Introduction
A diverse variety of benign and malignant lesions can be found in the colorectum. The vast majority of these are of epithelial origin, such as tubulo/villous or serrated adenomas, hyperplastic polyps, juvenile polyps, hamartomas and neuroendocrine tumors. Mesenchymal tumors in the colon and rectum are quite rarely found. These include GIST, leiomyomas, lipomas, schwannomas, perineurinomas, ganglioneuromas, granular cell tumor, and different kinds of vascular tumors (1).
The histological diagnosis of lipomas is usually straightforward capturing the mature fat cells that proliferate in a benign manner, sometimes accompanied by thick-walled and dilated vessels. The first colonic lipoma diagnosis dated back to 1757 by Bauer. Among benign
Figure 1: Endoscopic appearance of the long pedunculated polyp which retrack into the terminal ileum through the Bauhin’s valve
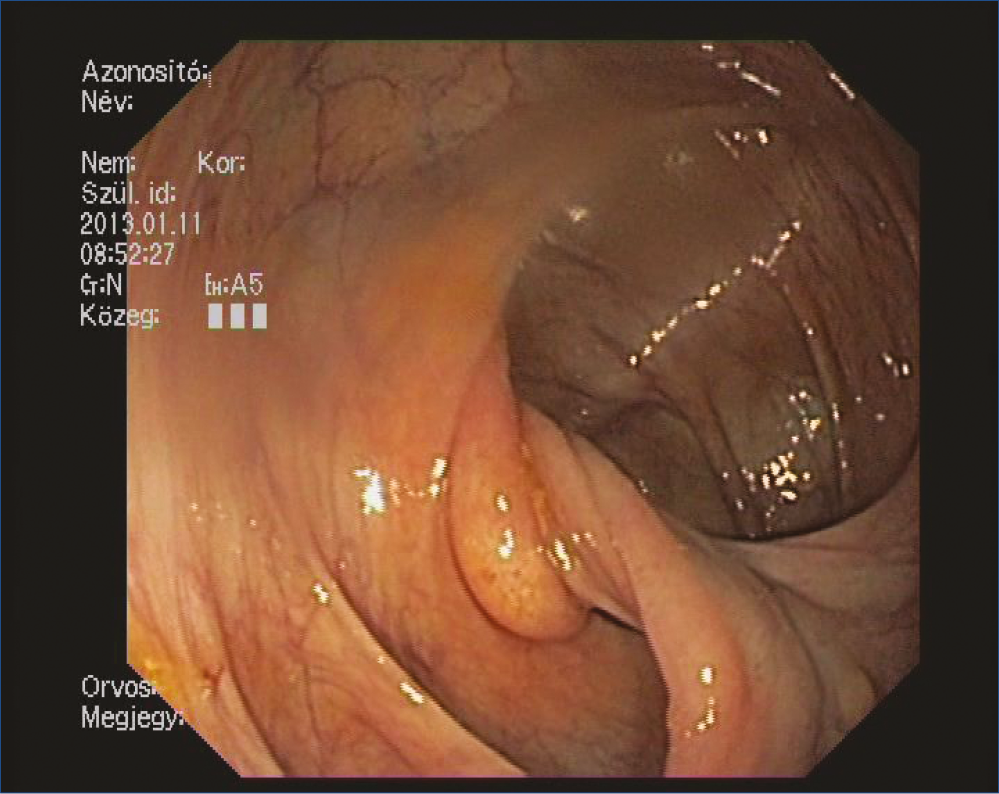
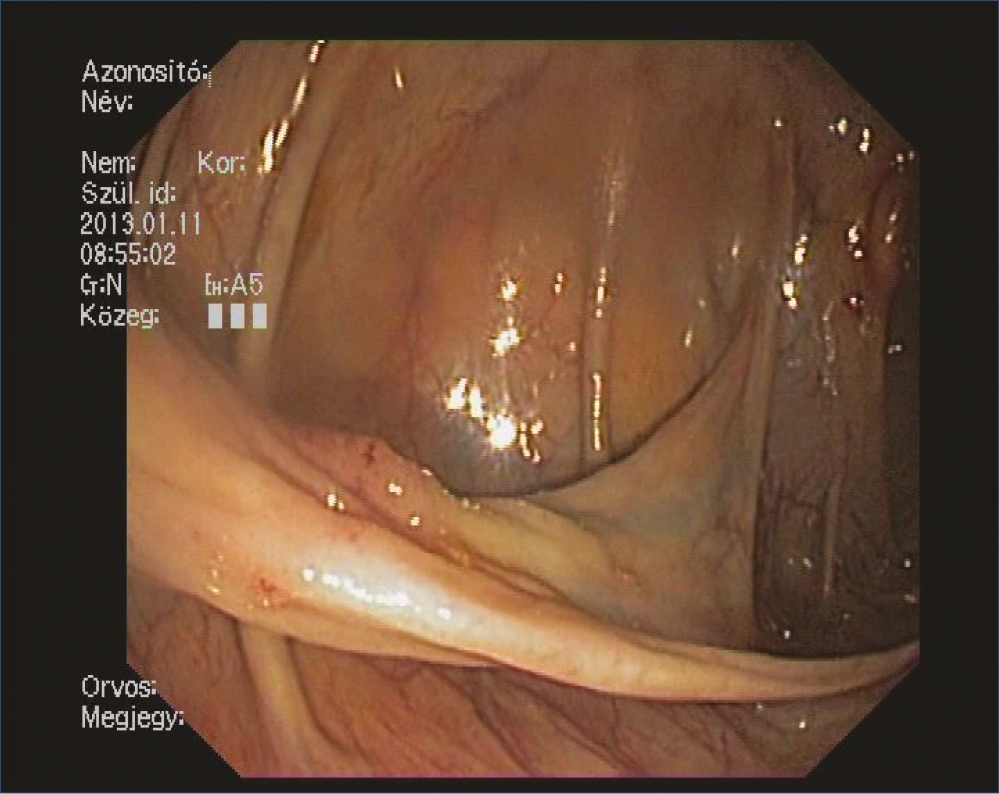
Figure 2: 30 mm long and tender lipoma which possessed a wide and crass vessel containing stalk
epithelial tumors, lipomas are the most common ones (2) with an incidence among the polypoid lesions of the colon ranging from 0.035% to 4.4% (3, 4). Lipomas can be found usually after the 5th decade and can locate anywhere along the GI tract; but, up to 50-90% of cases are located in the right side of the colon (4, 5) and 10% can be multiple (6). Some studies found a slight female predominance (7, 8).
Lipomas usually form a polypoid mass in the GI-tract and potentially mimic the more commonly found epithelial counterparts that might reveal a malignant potential latter.
Therefore, lipomas might cause severe differential diagnostic problems. Basically, the usual roundish shape of the lipomas suggests a benign behaviour; nevertheless, bigger lipomas often harbour profound secondary changes in the body and overlying mucosa such as inflammation, ulceration and necrosis. As polypoid tumors of the GI-tract are usually an indication of an endoscopic biopsy, a superficial sampling of the mucosa covering the lipoma might
Figure 3: The stalk of the lipoma contains wide and crass vessel
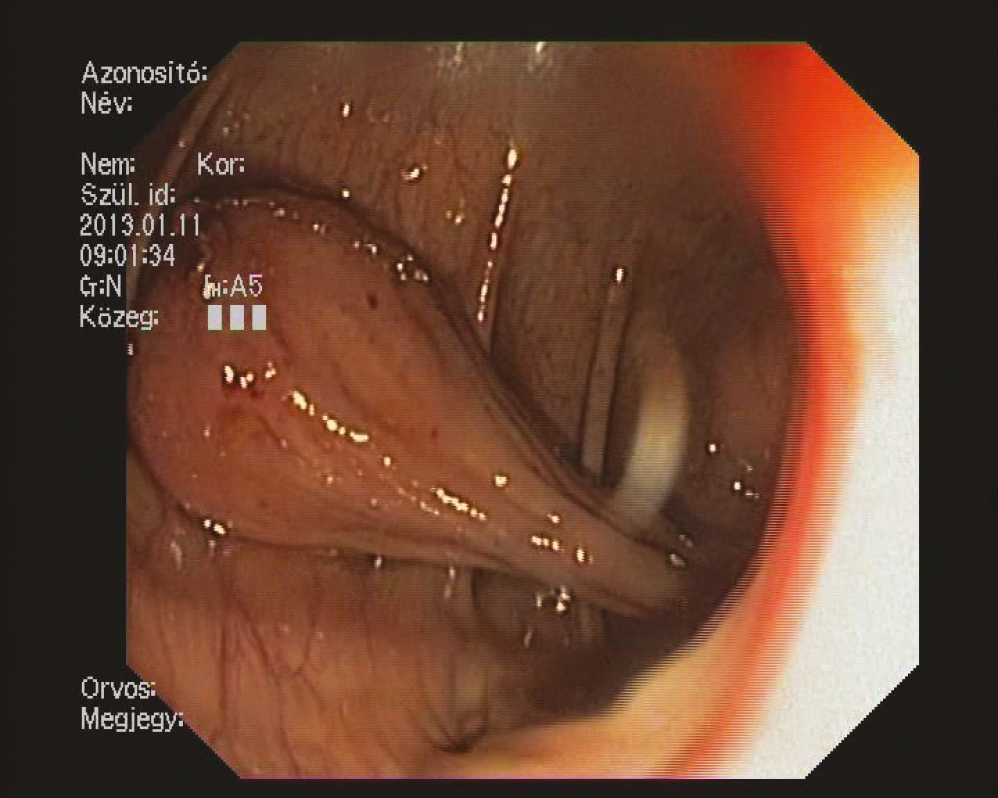
lead to even more differential diagnostic pitfalls, which might be a misdiagnosis/misinterpretation when the sample does not contain enough fatty tissue. In our case report of a relatively big lipoma in the cecum, we would emphasize on the differential diagnostic problems and sampling issues.
Case report
Screening colonoscopy was performed on a 69 year-old woman. The endoscopy examination revealed a long pedunculated polyp which grew back to the terminal ileum through the Bauhin fold, dividing it into two parts (Figure 1). The long polyp could be pulled back to the colon with a biopsy forceps. An 80 mm long and tender formation became visible which possessed a wide and crass vessel containing, stalk (Figure 2, 3). Due to the tenderness, our initial diagnosis was essentially a lipoma. We performed the polypectomy 4 days later: in order to prepare for an incidental complication, on-site surgical intervention was facilitated. After endo-loop placement onto the stalk, the polyp was removed entirely without complication.
Figure 4: Lipoma’s HE stain at low magnification. Under the mucosa the area is filled with “empty-looking” cells, adipocytes with some septa and thick-walled vessels
Figure 5: Lipoma stalk, vessels, surface arborization. The stalk of the lipoma contains adipocytes and dilated, thick-walled vessels. Surface shows elongated and bifurcating crypts
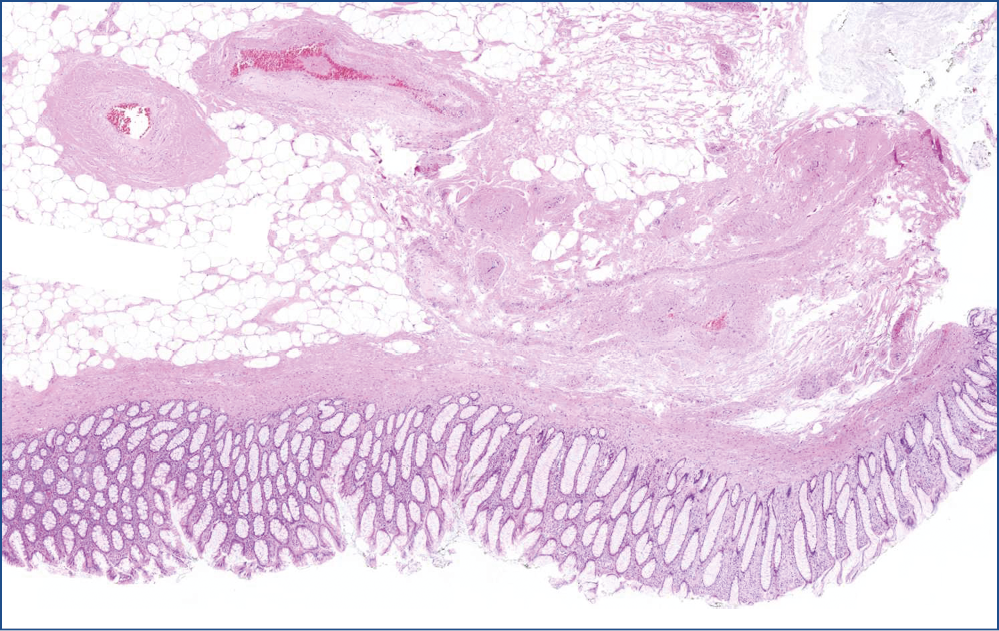
A 37 mm tumor was prepared for the pathological investigation. The surface was smooth with areas of some irregularity and red discoloration. The 20×10×8 mm stalk was also separately examined. Both stamples appeared to be composed of mature fatty tissue, under the naked eyes.
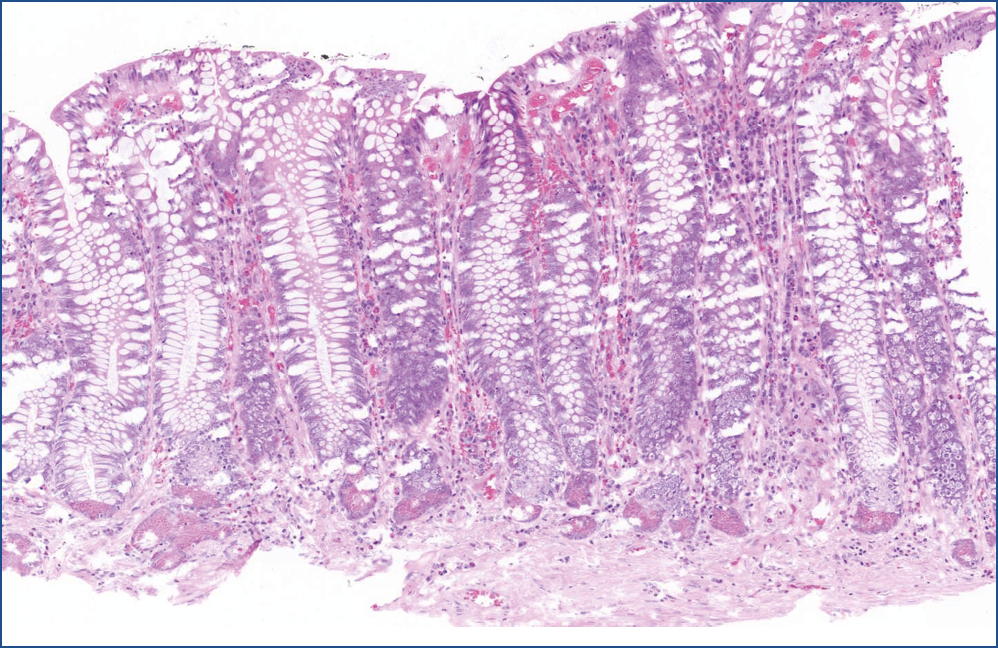
Microscopic evaluation revealed that, the polyp consisted of mature fatty tissue (Figure 4), which was divided by fibrous septa and characteristically thick-walled, dilated vessels (Figure 5). The border of normal submucosa was not identifiable. On the surface, the mucosa was of colonic type, but there was a visible nodule in which several secondary changes were recognized. That is, disseminations by erosion and a mixed type inflammatory reaction were present in the lamina propria and also partly infiltrated into the crypts (Figure 6). Along with this inflammatory reaction and erosion, the crypts showed a relatively minimal decrease in mucin production. Moreover, other elements of atypical inflammation/regeneration have also been observed: (1) nuclear crowding and nuclear activation, and (2) an increased amount of leukocytes among the epithelial cells (this could also be seen in the lamina propria) (Figure 7). A substantial count of Paneth cells was noted elongated structures found in other areas of the crypts (Figure 8), whilste others showed some serration towards their surface, resembling a picture of a hyperplastic polyp (Figure 5).
In conclusion, the histopathological diagnosis was that of a pedunculated submucosal lipoma. The size and the pedunculation caused a chronic movement (the polyp was found to be translocated into the terminal ileum) and irritation to the mucosa of the polyp, resulting in secondary changes: erosion, inflammations and a reactive hyperplastic reaction on its mucosal surface.
Discussion
Lipomas are typically growing out from the submucosa as sessile polypoid mass, but some can be pedunculated. About 75% of lipomas are asymptomatic and found incidentally at surgery, autopsy or colonoscopy, especially fewer than 2 cm of diameter (2, 4, 8–10).
Figure 6: Lipoma nodule. A nodule with transformed mucosa: crypts show decrease in mucin production, dilation, variation in size and shape and is partly replaced by granulation tissue (right)
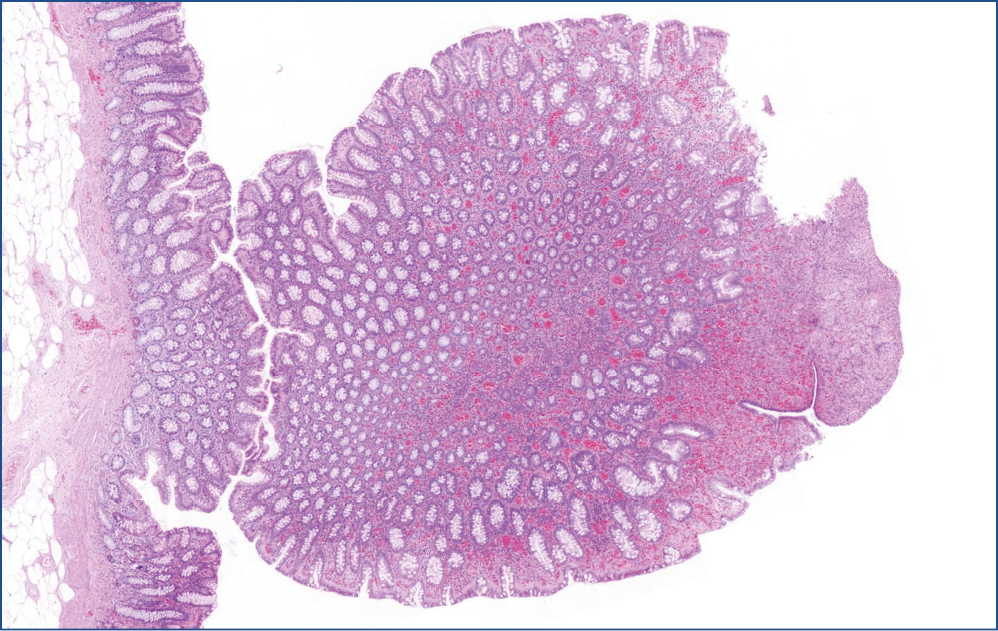
Figure 7: Lipoma erosion, close up of the nodule. Erosion is observed on the right sided areas that were replaced by granulation tissue, whereas on the left side, the mucosa became quite loose, because of the heavy mixed inflammatory cell infiltration. At the same time, crypts showed a minimal decrease in mucin production and variation in shape and size, indicating atypical inflammation/regeneration
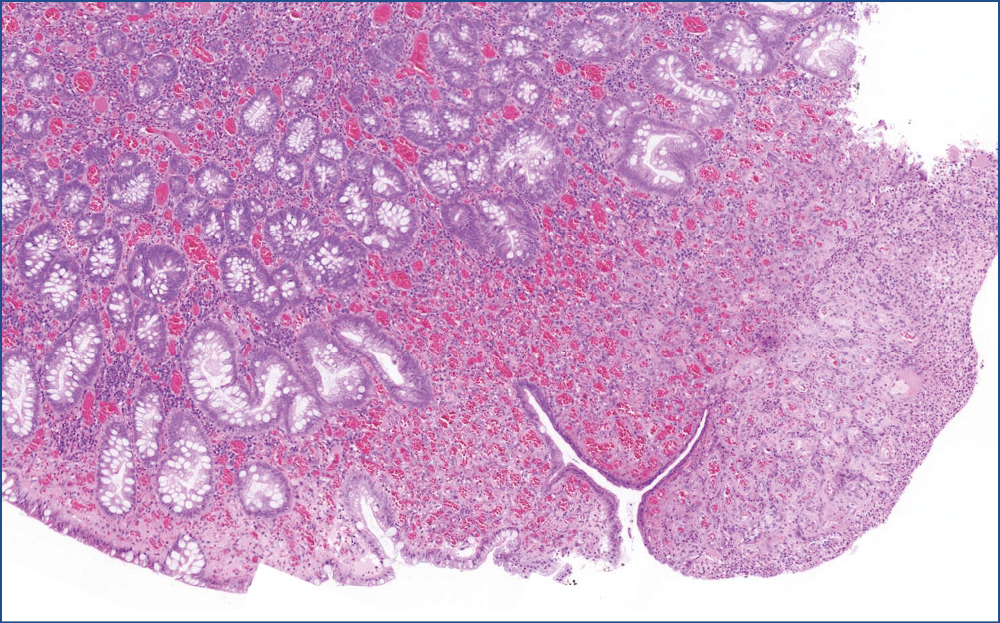
Figure 8: Hyperplastic crypts. The crypts became elongated with numerous Paneth-cells at the bottom. Towards the surface, crypts are a bit dilated and became uneven
Clinical manifestations are associated with the size of tumor, and usually only bigger lipomas would cause clinical symptoms. These can be abdominal pain, bleeding or melena, intermittent diarrhoea or alteration of bowel habits, obstruction of the passage or even intussusception and thus ileus (7). A recent review has found that lipoma is the most common benign tumor causing colonic intussusception in adults (11). Larger lipomas can be also self-amputated and appear as a lump of haemorrhagic tissue in the stool (8).
As lipomas occur quite rare, they are usually undermined and misdiagnosed for more serious pathological differential diagnoses. This causes potential problems since the exclusion of malignant lesions are the most relevant. Imaging techniques usually show a round and smooth contoured filling defect, a non-specific finding occurring in any kind of tumors. Lipomas have a so called characteristic sign with barium enema, the ’squeeze-sign’, depicting that the tumor can be deformed by pressure or peristalsis (6). CT is a very helpful tool because fatty tissue has a characteristic densitometric value (5, 11). Sometimes ultrasound can also correctly diagnose lipoma (12). Larger lipomas develop numerous fibrous intervals in their otherwise mature fatty tissue, giving a lobulated appearance (13). The bigger the lipoma is, the more secondary changes can occur such as fat-necrosis, inflammation, ulceration and granulation tissue formation. Thus, the normal histological and imaging picture is subjected to change, causing even more severe differential diagnostic problems possibly towards any malignancy (14, 15).
Colonoscopy is a very sensitive and widely used method for diagnosing lipomas and the colonoscopic picture of lipomas has several specific characteristic features like “ pillow or cushion sign”, the “naked fat sign”, and the “tenting effect” (4, 16–18). On the other hand, the Bauhin’s valve might appear to be bigger, because of fatty tissue content, which can be misdiagnosed for a lipoma. As fat is a normal component of the submucosa, benign findings are sometimes not enough to exclude any malignancy. Furthermore, as larger lipomas can develop secondary changes resulting in atypical structures (fibrosis, ulceration etc.), biopsy can be misleading. In summary, colonoscopic biopsy evaluation is not recommended, because of possible non-specific findings, due to low tissue yield and higher risk of bleeding or perforation (6). Thus, exact diagnosis relies on pathological examination of the entire resected polyp.
In case of smaller and asymptomatic lipomas, the clinical management ’watch and wait’ could be enough. Treatment options for lipomas with clinical symptoms are various. The smaller and pedunculated ones can be easily removed via colonoscopy, but the sessile or larger than 2 cm ones are subjected to an elevated risk of complications (e.g. more bleeding due to less effective cauterization and/or conductance in the fatty tissue) and, therefore, surgical resection is suggested (13, 19–21). Recently laparoscopic resection became more successful, but this needs a very strict and precise preoperative diagnosis (22, 23).
Histopathological diagnosis of lipomas can be done usually without any problem if the tumor is resected entirely, since the characteristic mature adipose tissue, thick-walled vessels are easy to recognize. The problem arises when the tumor is not entirely resected or sampling is superficial, and escalates when bigger tumors possess secondary changes in their adipose part (necrosis, bleeding especially when resection is incomplete, etc.) or on the surface due to the chronic irritation and movement of the polypoid mass.
Our case illustrated several interesting features. Firstly, during endoscopic investigation, only the long stalk became visible at first glance; and, the head had to be pulled back from the terminal ileum for better visualization. This suggested a probability or a potential risk of intussusception or occlusion of the bowel, justifying the need for resection. Usually, lipomas bigger than 2 cm are rather surgically removed, but the long stalk in our case made endoscopic removal with an endo-loop possible. On the other hand, the long stalk made the pedunculated lipoma mobile and prone to chronic irritation. The structure and endoscopic appearance were suggestive of lipoma, excluding the ambiguous irregular and reddish surface. This latter was proven to be a regenerative and hyperplastic change by microscopic examination.
Hyperplastic polyps are usually developed as reactive processes in the colon. For example, it might be a reactive change of the mucosa with a long history of chronic irritation and inflammation. As bigger lipomas are prone to harbour secondary changes on their surface (like erosion, ulceration and inflammation) due to the chronic irritation, it is understandable, that our lipoma bore hyperplastic features in the overlying mucosa, although this is very rarely reported in the literature (15). We hypothesized that this kind of hyperplastic change might be more commonly found than reported. Nonetheless, on the surface of larger lipomas, the chronic irritation rather causes ulcerations or even necrosis and auto-amputation (which could have presumably occurred with this lipoma later), and, at that point, the hyperplastic nature of the surface might not be further recognized. According to our hypothesis, this medium sized lipoma (4 cm) was big enough to develop a hyperplastic surface, but was not big enough to be totally ulcerated so this intermediate phenomenon with both hyperplastic and erosive signs on its surface could be harvested.
Conclusion
In summary, the most important challenge of colonic lipoma characterization is their differential diagnostic misleading caused by the polypoid appearance mimicking other malignancies or epitheloid tumors with a malignant potential. Our case also demonstrates that superficial sampling could have led to the possible misdiagnosis of a hyperplastic polyp or any kind of (ulcerative) colitis, therefore further highlights the need for complete resection and histological examination.
2. Atmatzidis S, Chatzimavroudis G, Patsas A, Papaziogas B, Kapoulas S, Kalaitzis S, Ananiadis A, Makris J, Atmatzidis K. Pedunculated cecal lipoma causing colo-colonic intussusception: a rare case report. Case Rep Surg 2012; 2012: 279213. [PMID: 23259130 PMCID: 3521399
doi: https://doi.org/10.1155/2012/279213 (doi)]
3. Castro EB, Stearns MW. Lipoma of the large intestine: a review of 45 cases. Dis Colon Rectum 1972; 15(6): 441–444
[PMID: 4645612]
4. Ryan J, Martin JE, Pollock DJ. Fatty tumours of the large intestine: a clinicopathological review of 13 cases. Br J Surg 1989; 76(8): 793–796.
[PMID: 2670056]
5. Liessi G, Pavanello M, Cesari S, Dell’Antonio C, Avventi P. Large lipomas of the colon: CT and MR findings in three symptomatic cases. Abdom Imaging 1996; 21(2): 150–152
[PMID: 8661762]
6. Zhang H, Cong JC, Chen CS, Qiao L, Liu EQ. Submucous colon lipoma: a case report and review of the literature. World J Gastroenterol 2005; 11(20): 3167–3169
[PMID: 15918213]
7. Rogy MA, Mirza D, Berlakovich G, Winkelbauer F, Rauhs R. Submucous large-bowel lipomas–presentation and management. An 18-year study. Eur J Surg 1991; 157(1): 51–55.
[PMID: 1675882]
8. Radhi JM. Lipoma of the colon: self-amputation. Am J Gastroenterol 1993; 88(11): 1981–1982
[PMID: 8237965]
9. Kim CY, Bandres D, Tio TL, Benjamin SB, Al-Kawas FH. Endoscopic removal of large colonic lipomas. Gastrointest Endosc 2002; 55(7): 929–931.
[PMID: 12024158 doi: https://doi.org/S0016510702868523 (pii)]
10. Pfeil SA, Weaver MG, Abdul-Karim FW, Yang P. Colonic lipomas: outcome of endoscopic removal. Gastrointest Endosc 1990; 36(5): 435–438.
[PMID: 2227312]
11. Chiang JM, Lin YS. Tumor spectrum of adult intussusception. J Surg Oncol 2008; 98(6): 444–447.
[PMID: 18668640 doi: https://doi.org/10.1002/jso.21117 [doi]]
12. Alkim C, Sasmaz N, Alkim H, Caglikulekci M, Turhan N. Sonographic findings in intussusception caused by a lipoma in the muscular layer of the colon. J Clin Ultrasound 2001; 29(5): 298–301.
[PMID: 11486326 doi: https://doi.org/10.1002/jcu.1038 [doi]]
13. Jiang L, Jiang LS, Li FY, Ye H, Li N, Cheng NS, Zhou Y. Giant submucosal lipoma located in the descending colon: a case report and review of the literature. World J Gastroenterol 2007; 13(42): 5664–5667.
[PMID: 17948945]
14. Pereira JM, Sirlin CB, Pinto PS, Casola G. CT and MR imaging of extrahepatic fatty masses of the abdomen and pelvis: techniques, diagnosis, differential diagnosis, and pitfalls. Radiographics 2005; 25(1): 69–85.
[PMID: 15653588DOI: https://doi.org/25/1/69 [pii] doi: https://doi.org/10.1148/rg.251045074 (doi)]
15. Radhi JM, Haig TH. Lipoma of the colon with overlying hyperplastic epithelium. Can J Gastroenterol 1997; 11(8): 694–695.
[PMID: 9459050]
16. De Beer RA, Shinya H. Colonic lipomas. An endoscopic analysis. Gastrointest Endosc 1975; 22(2): 90–91.
[PMID: 1193347]
17. Notaro JR, Masser PA. Annular colon lipoma: a case report and review of the literature. Surgery 1991; 110(3): 570-572
[PMID: 1887386]
18. Messer J, Waye JD. The diagnosis of colonic lipomas – the naked fat sign. Gastrointest Endosc 1982; 28(3): 186–188.
[PMID: 7129044]
19. Katsinelos P, Chatzimavroudis G, Zavos C, Pilpilidis I, Lazaraki G, Papaziogas B, Paroutoglou G, Kountouras J, Paikos D. Cecal lipoma with pseudomalignant features: a case report and review of the literature. World J Gastroenterol 2007; 13(17): 2510–2513.
[PMID: 17552037]
20. Yarze JC. Colonoscopic resection of an asymptomatic colon lipoma. Gastrointest Endosc 2006; 63(6): 890–891; author reply 891–892.
[PMID: 16650574 doi: https://doi.org/S0016-5107(05)03416-4 [pii] 10.1016/j.gie.2005.12.011 (doi)]
21. Raju GS, Gomez G. Endoloop ligation of a large colonic lipoma: a novel technique. Gastrointest Endosc 2005; 62(6): 988–990. [PMID: 16301055 doi: https://doi.org/S0016-5107(05)02658-1 [pii] 10.1016/j.gie.2005.08.018 (doi)]
22. Ladurner R, Mussack T, Hohenbleicher F, Folwaczny C, Siebeck M, Hallfeld K. Laparoscopic-assisted resection of giant sigmoid lipoma under colonoscopic guidance. Surg Endosc 2003; 17(1): 160. [PMID: 12399859 doi: https://doi.org/10.1007/s00464-002-4232-3 [doi]]
23. Peters MB, Jr., Obermeyer RJ, Ojeda HF, Knauer EM, Millie MP, Ertan A, Cooper S, Sweeney JF. Laparoscopic management of colonic lipomas: a case report and review of the literature. JSLS 2005; 9(3): 342–344.
[PMID: 16121884 PMCID: 3015622
