Eredeti közlemény / Original paper
Combination therapy with anti-TNFs and thiopurines does affect drug metabolite levels but it is not associated with body composition in inflammatory bowel disease patients: A cross-sectional study
Summary
In this cross-sectional, real-life study we have investigated the potential association between 6-thioguanine nucleotide (6-TGN) and anti-TNF [infliximab (IFX), adalimumab (ADA)], anti-drug antibody levels and body composition parameters. Based on our results thiopurine and anti-TNF combination therapy resulted in decreased antibody formation in IFX-treated patients. AZA-ADA-treated patients showed increased anti-TNF drug concentrations, regardless of antibody formation. Drug metabolites did not correlate with body composition parameters.
Ezen keresztmetszeti vizsgálat során a 6-tioguanin nukleotid szintje (6-TGN), az anti-TNF (infliximab [IFX], adalimumab [ADA]), a gyógyszerellenes antitestek és a testösszetétel-paraméterek közötti lehetséges összefüggéseket vizsgáltuk. Eredményeink alapján azatioprin és IFX kombinációs terápia esetén alacsonyabb a gyógyszerellenes antitestek szintje. Azatioprin és ADA kombinációs terápia esetén magasabb ADA-szint volt kimutatható, míg a gyógyszerellenes antitestek szintje nem változott. A vizsgált gyógyszer- szintek nem mutattak összefüggést a mért testösszetétel-paraméterekkel.
Introduction
The medical management of patients with inflammatory bowel disease (IBD, Crohn’s disease [CD], ulcerative colitis [UC]) is determined by the location, behaviour and activity of the disease. The therapeutic strategy is also influenced by previous treatment response, possible side effects, steroid dependence or refractoriness and the presence of extra-intestinal manifestations or complications. For over 50 years, thiopurines, such as azathioprine (AZA) and 6-mercaptopurine (6-MP) have been used for the effective treatment of steroid-dependent IBD patients and that of refractory disease. Anti-TNF treatment is reserved for steroid-dependent or steroid- or immunomodulator-refractory patients (1). Several studies have demonstrated the clinical importance of therapeutic drug monitoring (TDM), an important tool that has been proven to optimise anti-TNF therapy effectively. TDM may help to better understand and manage unfavourable therapeutic outcomes, which are most commonly associated with immunogenicity and/or low drug concentrations during anti-TNF treatment (2–6). However, the TDM of thiopurines has not been applied in daily clinical practice despite the long-term use of these drugs. Clinical data suggest that there is a synergistic relationship between AZA and infliximab (IFX). The underlying mechanisms of this effective combination include a simultaneous increase in the therapeutic effectiveness and a decrease in the rate of secondary loss of response associated with immunogenicity and the formation of antibodies against anti-TNF agents (7–9). However, data regarding the influence of thiopurines on the pharmacokinetics of anti-TNF therapy, particularly that of adalimumab (ADA), is limited. Although body composition analysis may change our perspective on AZA dosing, data regarding the influence of patients’ nutritional status on thiopurine metabolism is limited and controversial. Considering the complex metabolic pathways of these drugs, their relatively narrow therapeutic window and the differences in tolerance among patients, optimal dosing may be difficult to achieve. We aimed to measure serum anti-TNF and 6-thioguanine nucleotide (6-TGN) levels in our IBD patients receiving maintenance anti-TNF and/or thiopurine therapy to investigate how AZA and anti-TNF combination therapy affect serum drug and AZA metabolite levels. Similarly to most IBD centres we did not have the possibility to measure AZA metabolite level before this study. Therefore, we also aimed to correlate 6-TGN and anti-TNF levels with the outcome of our routinely used, symptom-based therapeutic optimalisation. We wanted to find out how many patients who received thiopurine treatment with the conventional administration would present subtherapeutic 6-TGN levels and require dose escalation. Furthermore, we aimed to evaluate the correlation between 6-TGN blood levels and anti-TNF trough levels and bodyweight, body-surface area and different body composition parameters.
Materials and methods
Patient population
This cross-sectional study included consecutive CD and UC patients treated with maintenance AZA monotherapy or AZA and anti-TNF combination therapy (AZA-IFX and AZA-ADA combination therapy) at the Department of Medicine, University of Szeged. Activity index-based pair-matched, randomly selected control patients receiving anti-TNF monotherapy were included in the control group. The 6-TGN levels of outpatients on AZA mono-, or combination therapy were measured without any change to the prior therapy. Written, informed consent was obtained from all participants included in the study. In Hungary, the application of biological treatment is strictly regulated by the National Health Insurance Fund. The administration of a thiopurine drug is mandatory for at least 3 months prior to the start of anti-TNF treatment, except for acute, severe flare-ups. Therefore, patients on anti-TNF monotherapy are usually intolerant to thiopurines or they had a severe flare-up before the initiation of anti-TNF therapy. Moreover, certain comorbidities or clinical situations may also lead to the discontinuation of immunomodulatory therapy, which falls under the competence of the attending physician. The dosing of thiopurines and anti-TNF agents is based on the international guidelines, Hungarian financial protocols, patients’ tolerance, gastroenterologists’ decision and the risk stratification of patients for a more aggressive
disease phenotype. IFX and ADA maintenance therapies were defined as 14-plus weeks after initiating the therapy, with no interval between maintenance infusions >8 weeks for IFX and 2 weeks for ADA. Clinical data were collected; blood samples were also obtained to determine thiopurine metabolites, anti-TNF trough levels and antibody concentrations. The blood samples were stored at −20°C.
Clinical evaluation
Treatment outcomes were assessed during sampling and they were classified as clinical remission according to
disease activity scores (CDAI <150 or pMayo score ≤2) (10, 11). The following independent variables were considered: demographics, disease type and treatment duration, disease activity scores (pMayo, CDAI), BMI and body composition parameters. Furthermore, anti-TNF trough level, anti-TNF antibody and 6-TGN levels were obtained. For statistical analysis, 6-TGN and anti-TNF trough levels were classified as subtherapeutic, therapeutic or supratherapeutic. The therapeutic range was stratified according to literature data, 235–450 pmol/8×108 RBC for 6-TGN, 3–8 µg/ml for IFX and 5–12 µg/mL for ADA (2, 12–14).
Measurement of thiopurine metabolite concentration
6-TGN levels were measured using high-performance liquid chromatography (HPLC) (15). Whole blood samples anticoagulated with sodium heparin were used. 6-TGN and 6-MMP were separated on Agilent 1200 HPLC. Thiopurine metabolites were determined using UV detectors at 341 nm for 6-TGN. 6-MMP concentration could not be measured.
Measurement of anti-TNF and antidrug antibody concentration
The serum IFX (cat. No.: TR-Q-INFLIXIv2) and ADA (cat. No.: TR-ADAv1) concentrations were determined using the ELISA as per the manufacturer’s protocol (Matriks Biotek Laboratories, Ankara, Turkey). The sensitivity of the IFX and ADA assays was 30 ng/mL and 10 ng/mL, respectively. The intra- and inter-assay coefficients of variation for both the assays were <20%. The levels of antibodies for IFX (cat. No.: TR-ATIv5) and ADA (cat. No.: TR-AADAv2) in the serum was determined using ELISA assay as per the manufacturer’s protocol (Matriks Biotek Laboratories, Ankara, Turkey). The sensitivity of the anti-IFX and anti-ADA kits was 5 ng/mL and <30% ng/mL, respectively. The intra- and inter-assay coefficients of variation of both assays were <15%. Anti-TNF antibody concentrations were assessed in all patients receiving anti-TNF therapy.
Body composition analysis
Bioelectrical impedance analysis was performed using the InBody 770® analyser. This method measures total body water, extra- and intracellular water, skeletal muscle mass, body and visceral fat mass, percent body fat, protein and mineral levels, bone mineral content and body mass index (BMI). Normal BMI was assumed at 18.5–25 kg/m2, and the body surface area was calculated using the Mosteller formula. The Mosteller formula is the following:
√([height (cm) × weight (kg)]/3600)
Statistical analysis
Statistical analysis was performed using STATA 9.0 and SPSS. Continuous variables are presented as mean (minimum–maximum) values, while categorical variables are presented as counts (percentages). Continuous variables of two groups were compared with the Mann-Whitney U-test; in case of more than two groups the Chi-squared test was used, and correlations between continuous variables were investigated with the Spearman correlation.
Ethical approval
This study was approved by the Human Investigation Review Board of the University of Szeged Faculty of Medicine Albert Szent-Györgyi Clinical Centre (license number: 4126). The study conforms to the Declaration of Helsinki. All participants gave an informed, written consent prior to their inclusion in the study.
Results
Demographic data
In total, 163 consecutive IBD patients were enrolled. The baseline characteristics of these patients are shown in Table 1. Among the enrolled patients, 65 received AZA monotherapy and 49 received AZA and anti-TNF combination therapy (28 received IFX and 21 received ADA). Forty-nine activity index-based pair-matched control patients receiving anti-TNF monotherapy were included in the control group. Among the enrolled patients 12.3% had active
disease at the time of enrolment.
Table 1. Baseline characteristics of patients receiving AZA or AZA-anti-TNF combination or anti-TNF monotherapy (control group)
6-TGN concentration
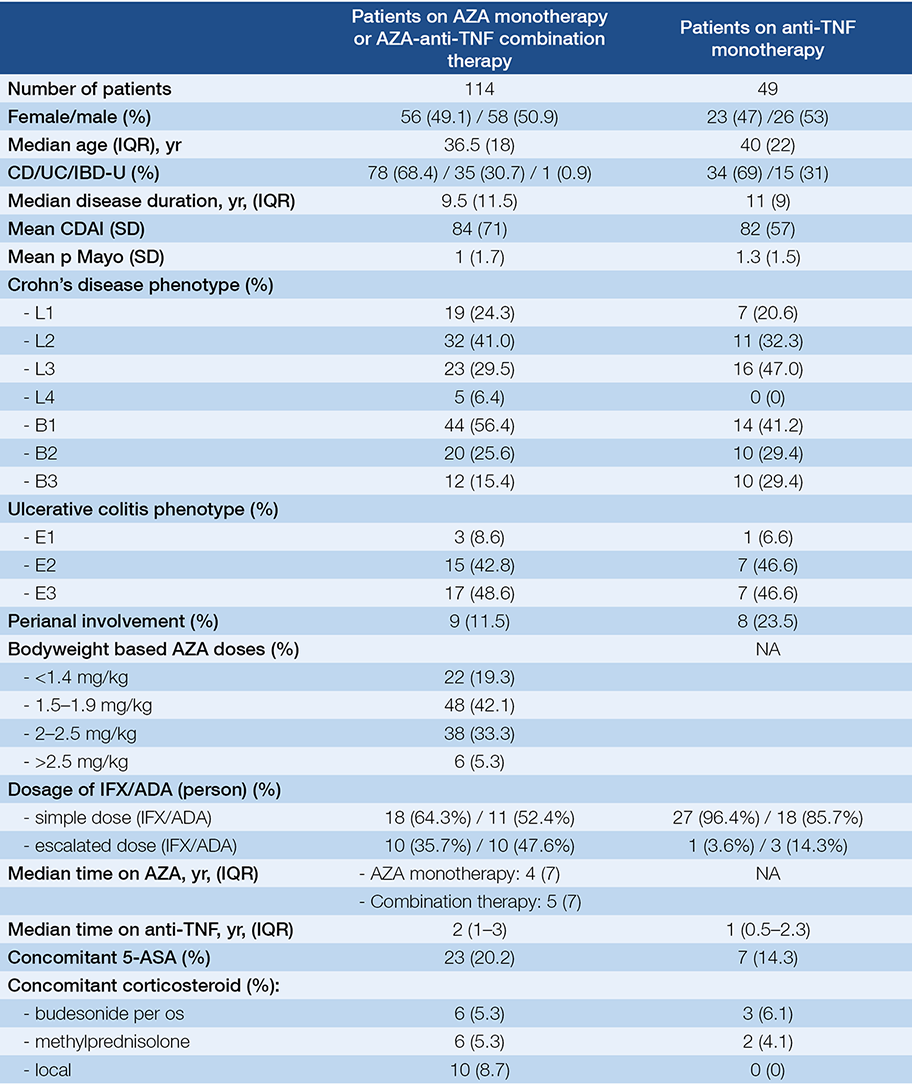
Forty-four point seven % of all enrolled patients had therapeutic 6-TGN levels with the conventional AZA dosage, 19.3% had sub-, and 36% had supratherapeutic 6-TGN levels. In case of AZA monotherapy and AZA-anti-TNF combination therapy 38.5% and 51% had therapeutic 6-TGN levels, 13.8% and 26.5% had subtherapeutic, while 47.7% and 22.4% had supratherapeutic 6-TGN levels (P=0.017). The 6-TGN concentration was found to be significantly lower in patients receiving AZA-anti-TNF combination therapy (397 [117–1250] pmol/8×108 RBC), compared to AZA monotherapy (619.3 [128–3875] pmol/8×108 RBC; P=0.003). Mean bodyweight-based AZA doses were 1.7 mg/kg (0.3–3.2) in patients treated with AZA monotherapy and 1.8 mg/kg (0.4–3.3) in patients receiving AZA-anti-TNF combination therapy (P=0.1). The bodyweight-based AZA doses in both patient groups are presented in Fig. 1. Eighty-seven point eight % of the patients in the combination group and 87.7% of the patients in the AZA monotherapy group were in sustained remission. The mean concentration of 6-TGN did not differ between patients receiving AZA-IFX and AZA-ADA combination therapies (385 [117–1250] pmol/8×108 RBC and 412.8 [122–1088] pmol/8×108 RBC), respectively (P=0.77).
Fig. 1. Body weight-based AZA doses separately in patients on AZA monotherapy and combined AZA- anti-TNF therapy
Anti-TNF trough levels
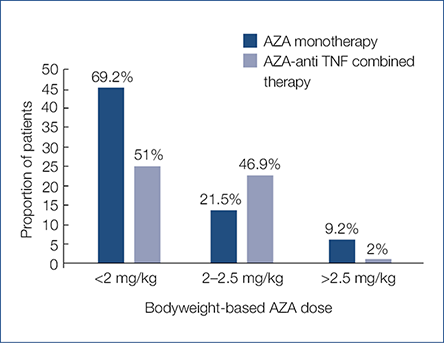
The IFX concentration did not differ between the AZA-IFX combination therapy and the IFX monotherapy groups (10.4 [1.2–48.7] μmol/mL vs. 8 [0–31.7] μmol/mL [P=0.103]; Fig. 2). However, the proportion of patients with subtherapeutic IFX concentrations was lower in the combination therapy group compared to the IFX monotherapy group (17.8% vs. 53.6%; P=0.011). The concentration of ADA was higher in patients receiving combination therapy than in those receiving ADA monotherapy (16.8 [0–48.6] μmol/mL vs. 6.5 [0–16.6] μmol/mL, P=0.018; Fig. 2). The proportion of patients with subtherapeutic ADA levels in the monotherapy and combination therapy groups was 52.4% and 14.2%, respectively (P=0.02). 6-TGN concentrations and IFX or ADA trough levels showed no correlation (r = −0.06 and 0.09, respectively). An escalated dose of IFX was applied in 35.7% of AZA-IFX treated and 3.6% of IFX monotherapy treated patients (P=0.005). Escalated ADA was used in 47.6% of AZA-ADA and 14.3% of ADA treated patients (P=0.043). However, we did not find any significant differences in mean drug levels. In case of IFX monotherapy, only one patient received escalated IFX therapy, and we could not measure IFX drug level. In case of ADA monotherapy mean ADA concentration was 6.34 µg/mL (SD: 4.98) with normal ADA dose, and among those receiving escalated ADA therapy mean ADA concentration was 7.66 µg/mL (SD: 7.92), (P=0.803). In case of AZA-IFX combination therapy mean IFX concentration was 11.46 µg/mL (SD: 11.94) with normal IFX dose, and mean IFX concentration was 8.46 µg/mL (SD: 7.62) with escalated IFX dose (P=0.425). In case of AZA-ADA combination therapy mean ADA concentration was 10.72 µg/mL (SD: 8.07) with normal ADA dose, and mean ADA concentration was 23.58 µg/mL (SD: 18.38) with escalated ADA dose (P=0.064).
Anti-drug antibody level On examining the proportion of patients with detectable antibody levels regarding the type of anti-TNF agent, antibody positivity was detected in 7.1% vs. 42.8% of patients receiving AZA–IFX combination therapy and IFX monotherapy (P=0.004) (Fig. 3). Among ADA-treated patients, antibody positivity was found in 38.1% of patients receiving combination therapy and in 47.6% of those receiving monotherapy (P=0.756) (Fig. 3). Among patients receiving AZA–anti-TNF combination therapy, no difference in 6-TGN concentrations was found between those that developed anti-drug antibodies and those who did not (525 [122–1250] pmol/8×108 RBC and 364 [117–738] pmol/8×108 RBC; P=0.5).
Fig. 2. Mean IFX and ADA concentrations in patients receiving mono or combo therapy (P=0.376; P=0.007, respectively)
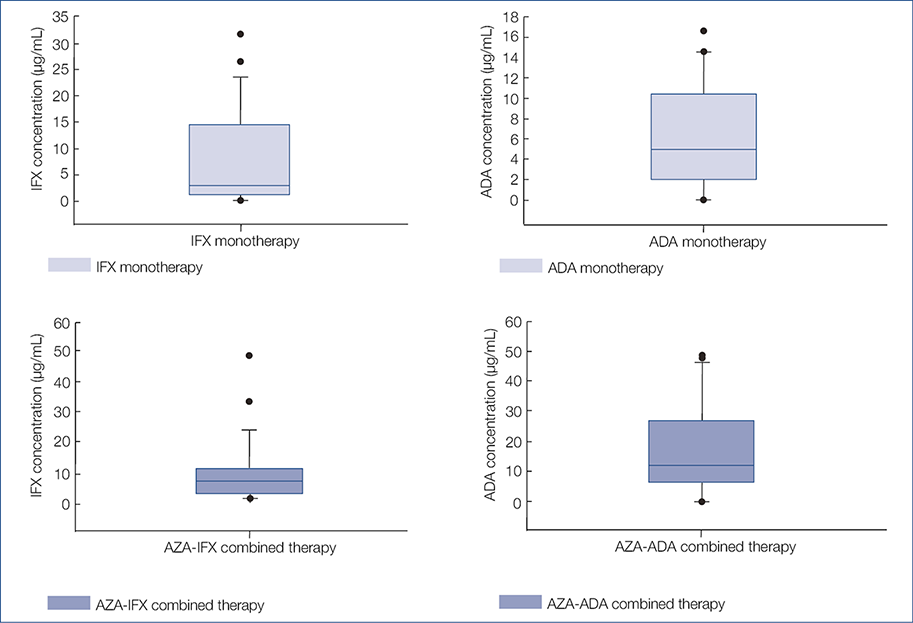
Fig. 3. Proportion of patients with detectable antibody levels
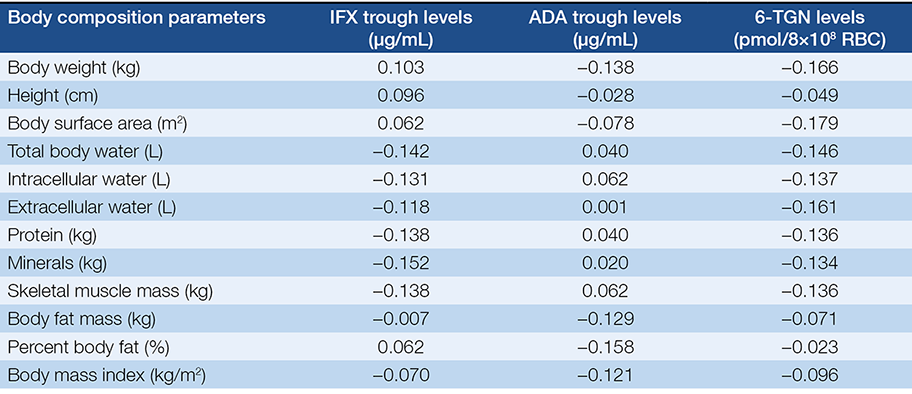
Correlation with body composition parameters
The body composition was determined in 114 patients. The BMI was low in 5.2% of the patients, normal in 44.7% and high in 49.1%. We examined the potential correlation of 6-TGN levels with bodyweight-based AZA dose, body surface area-based AZA dose, and different body composition parameters (Table 2.). A weak correlation was found between 6-TGN levels and bodyweight-based AZA doses (r=0.25; P=0.007) as well as body surface area-based AZA doses (r=0.22; P=0.017). However, none of the examined parameters correlated with 6-TGN levels. The body composition of 48 patients treated with AZA–anti-TNF combination therapy was determined. No correlations were found between the investigated body composition parameters and anti-TNF trough levels (Table 2.).
Table 2. Correlation between different body composition parameters („r” refers to Spearman’s rho)
Discussion
In the present observational, cross-sectional study, we evaluated 6-TGN and anti-TNF trough levels in 163 consecutive IBD patients receiving maintenance thiopurine monotherapy or combined thiopurine and anti-TNF therapy.
6-TGN concentrations mainly depend on individual enzymatic variations. Nevertheless, many other factors may influence the concentration, including age- and ethnicity-related difference in AZA metabolism, as well as concurrent drug therapy (16). The mean 6-TGN concentration was significantly lower in patients receiving AZA–anti-TNF combination vs. AZA monotherapy; however, this did not affect the rate of clinical remission, and the mean dose of AZA did not differ in the two groups. The exact reason for the lower 6-TGN level in patients treated with combined therapy is not clear; we assume that the anti-TNF effect might contribute to lower 6-TGN levels. However, no correlation was found between 6-TGN concentrations and IFX or ADA concentrations.
Currently, AZA monotherapy is recommended to be introduced gradually, generally by applying weight-based doses. The therapeutic effectiveness of AZA correlates with 6-TGN levels of 235–450 pmol/8×108 RBC (17, 18). Current results suggest that there is only a weak correlation between the level of 6-TGN and bodyweight-based AZA dose. However, bodyweight-based AZA did not correlate with other body composition parameters, except for body surface area. Almost forty-five % of the enrolled patients had therapeutic 6-TGN levels with the conventional AZA dosage, despite the fact that most patients receiving AZA, received an AZA dose less, than 2 mg/kg. Moreover, more than two thirds of the AZA monotherapy and AZA-anti-TNF combination therapy patients were in remission.
The superiority of the AZA–IFX combination therapy to the monotherapy of either drug has also been shown in large, clinical trials (19–21). However, in case of ADA, this beneficial effect is questionable (22). Our results correspond with a retrospective study evaluating the influence of immunomodulators on anti-TNF trough levels and antibody formation, which found no difference in antibody formation between ADA monotherapy and ADA-immunomodulator combination therapy. However, in case of IFX, antibody formation was found to be significantly lower in patients receiving combination therapy compared to IFX monotherapy (20). These results are consistent with previous studies by Holstrom et al. and Karmiris et al., which demonstrated that immunomodulators did not reduce the formation of antibodies against ADA (23, 24). A prospective, randomized trial found that both ADA monotherapy and AZA–ADA combination therapy have a similar effect on maintaining clinical remission in CD. However, the AZA–ADA combination therapy resulted in higher rates of endoscopic improvement at week 26th (25). In our cohort, the mean ADA concentration was significantly higher for the combination therapy, although a higher proportion of AZA-ADA treated patients received an escalated dose (47.6%) compared to ADA monotherapy patients (14.3%). The previously mentioned prospective, randomized trial reported a trend toward higher ADA concentration in the AZA-ADA combination therapy group (25). No such difference was found regarding IFX therapy, despite the higher proportion of AZA-IFX combination therapy patients receiving escalated IFX therapy (35.7%) compared to IFX monotherapy patients (3.6%); the favourable effect of the AZA-IFX combination was possibly caused by decreased antibody formation. Among patients treated with either IFX or ADA monotherapy, a significantly higher proportion developed subtherapeutic anti-TNF drug concentrations than those receiving AZA and anti-TNF combination therapy.
Because thiopurine and IFX doses are based on bodyweight, it is still unclear whether there is an association between the metabolism of thiopurine and IFX and body composition. We hypothesized that body mass and composition may influence anti-TNF and 6-TGN levels. No correlations were found between 6-TGN levels or anti-TNF trough levels and bodyweight, body surface area, total body water, intra- or extracellular water, protein, skeletal muscle mass and body fat mass. A weak correlation was found between 6-TGN levels and the bodyweight-based and body surface area-based AZA doses. The effectiveness and safety of thiopurines’ is known to depend on individual enzymatic variations. We could not confirm the influence of body composition.
The present study has some limitations that should be mentioned. First of all, this is a non-randomised, cross-sectional study enrolling patients already on maintenance anti-TNF therapy and/or AZA, which could have resulted in a selection bias. Some steps of the thiopurine metabolism were not investigated, including thiopurine S-methyl transferase genotyping, nucleoside diphosphate-linked moiety X motif 15 genotyping, and 6-MMP concentrations. However, the enrolled patients receiving AZA mono-, or combination therapy have no known thiopurine intolerance. The proportion of patients on escalated doses differed between patients receiving anti-TNF monotherapy and anti-TNF and thiopurine combination therapy. Although this did not affect the concentration of IFX between the two groups, higher ADA concentrations could be the consequence of the different proportion of patients on escalated ADA doses. Nevertheless, the proportion of dose escalation in this cohort follows the international trends and indicated only in case of loss of response. Sample and group sizes are too small to make strong conclusions. On the other hand, our study investigates data from real-life and adds to the existing body of information about AZA and ADA combination therapy.
Conclusion
Our data suggest that the possible synergistic effect of thiopurine and anti-TNF combination therapy is based on the decreased antibody formation in IFX-treated patients and increased anti-TNF drug concentration regardless of immunogenicity in ADA-treated patients. Similarly to previous studies, we failed to confirm/ detect a correlation between drug metabolites and different body composition parameters.
Acknowledgement
Disclosures: Károly Palatka received speaker’s honoraria from Takeda, AbbVie, Janssen, Ferring. Klaudia Farkas has received speaker’s honoraria from AbbVie, Janssen, Ferring, Takeda and Goodwill Pharma. Tamás Molnár has received speaker’s honoraria from MSD, AbbVie, Egis, Goodwill Pharma, Takeda, Pfizer and Teva.
Grant Support: This work was supported by the research grants of the National Research, Development and Innovation Office (Grant ID: 119809, 125377 and 129266) and by the EFOP-3.6.2-16-2017-00006 and by the University of Szeged Open Access Fund (4841).
Author’s contributions: conception and design of the study: Klaudia Farkas, Tamás Molnár, data collection: Kata Szántó, Anna Fábián, Renáta Bor, Anita Bálint, Mariann Rutka, Ferenc Nagy, Zoltán Szepes, Károly Palatka, Tibor Tóth, mea-
surement of thiopurine metabolite and anti-TNF levels: Zoltán András Mezei, Diána Kata, Imre Földesi, analysis and interpretation of the data: Kata Szántó, Tibor Nyári, drafting and revision of the manuscript: Anna Fábián, Ágnes Milassin, Klaudia Farkas, Tamás Molnár, approval of the final version of the manuscript: all authors.
2. Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol 2016; 7: 289–300.
https://doi.org/10.1136/flgastro-2016-100685
3. Steenholdt C, Brynskov J, Thomsen OO, Munck LK, Fallingborg J, Christensen LA et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014; 63: 919–27.
https://doi.org/10.1136/gutjnl-2013-305279
4. Vande Casteele N, Ferrante M, Van Assche G, Ballett V, Compernolle G, Van Steen K et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015; 148: 1320–9.e3. https://doi.org/10.1053/j.gastro.2015.02.031
5. Papamichael K, Juncadella A, Wong D, Rakowsky S, Sattler LA, Campbell JP et al. Proactive Therapeutic Drug Monitoring of Adalimumab Is Associated With Better Long-term Outcomes Compared With Standard of Care in Patients With Inflammatory Bowel Disease. J Crohns Colitis 2019; 13(8): 976–981. https://doi.org/10.1093/ecco-jcc/jjz018
6. Restellini S, Chao C, Lakatos PL, Aruljothy A, Aziz H, Kherad O et al. Therapeutic Drug Monitoring Guides the Management of Crohn’s Patients with Secondary Loss of Response to Adalimumab. Inflamm Bowel Dis 2018; 24(7): 1531–1538. https://doi.org/10.1093/ibd/izy044
7. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. https://doi.org/10.1056/NEJMoa0904492
8. Panaccione R, Ghosh S, Middleton S, Marquez JR, Scott BB, Flint L et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014; 146: 392–400 e3. https://doi.org/10.1053/j.gastro.2013.10.052
9. Baert F, Noman M, Vermeire S, Van Assche G, D’Haens G, Carbonez A et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003; 348: 601–608. https://doi.org/10.1056/NEJMoa020888
10. Best WR, Becktel JM, Singleton JW et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70: 439–444.
https://doi.org/10.1016/S0016-5085(76)80163-1
11. Schroeder KW et al. Coated oral 5-aminosalycilic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317: 1625. https://doi.org/10.1056/NEJM198712243172603
12. Al Hadithy AF, de Boer NK, Derijks LJ, Escher JC, Mulder CJJ, Brouwers JRBJ. Thiopurines in inflammatory bowel disease: pharmacogenetics, therapeutic drug monitoring and clinical recommendations. Dig Liver Dis 2005; 37(4): 282–97. https://doi.org/10.1016/j.dld.2004.09.029
13. Mazor Y, Almog R, Kopylov U, Ben Hur D, Blatt A, Dahan A et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther 2014; 40: 620–628. https://doi.org/10.1111/apt.12869
14. Yalchin M, Kamieniarz L, Cama R, Tribich S, Lake L, Joyce H et al. Measuring thioguanine nucleotide (6-TGN) levels and clinical response in IBD. BMJ 2018; 67: A101–A102.
15. Cangemi G, Barco S, Melioli G. A validated HPLC method for the monitoring of thiopurine metabolites in whole blood in paediatric patients with inflammatory bowel disease. Int J Immunopathol Pharmacol 2012; 25(2): 435–44. https://doi.org/10.1177/039463201202500213
16. Lim SZ and Chua EW. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front Pharmacol 2018; 9: 1107. https://doi.org/10.3389/fphar.2018.01107
17. Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol 2011; 17: 4166–4173. https://doi.org/10.3748/wjg.v17.i37.4166 The rest of the references can be found in the editorial office and on the website http://www.gastronews.hu.
18. Simsek M, Meijer B, Mulder CJJ, van Brodegraven AA, de Boer NKH. Analytical pitfalls of therapeutic drug monitoring of thiopurines in patients with inflammatory bowel disease. Ther Drug Monit 2017; 39: 584–588. https://doi.org/10.1097/FTD.0000000000000455
19. Yarur AJ, Kubiliun MJ, Czul F, Sussman DA, Quintero MA, Jain A et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol 2015; 13: 1118–1124 e3. https://doi.org/10.1016/j.cgh.2014.12.026
20. van Schaik T, Maljaars JP, Roopram RK, Verwey MH, Ipenburg N, Hardwick JCH et al. Influence of Combination Therapy with Immune Modulators on Anti-TNF Trough Levels and Antibodies in Patients with IBD. Inflamm Bowel Dis 2014; 20: 2292–2298. https://doi.org/10.1097/MIB.0000000000000208
21. Roblin X, Serre-Debeauvais F, Phelip JM, Bessard G, Bonaz B. Drug interaction between infliximab and azathioprine in patients with Crohn’s disease. Aliment Pharmacol Ther 2003; 18: 917–925. https://doi.org/10.1046/j.1365-2036.2003.01778.x
22. Wong DR, Pierik M, Seinen ML, van Bodegraven AA, Gilissen LPL, Bus P et al. The pharmacokinetic effect of adalimumab on thiopurine metabolism in Crohn’s disease patients. J Crohns Colitis 2014; 8: 120–128. https://doi.org/10.1016/j.crohns.2013.07.004
23. Holmstrom RB, Mogensen DV, Brynskov J, Ainsworth MA, Nersting J, Schmiegelow K et al. Interactions between thiopurine metabolites, adalimumab and antibodies against adalimumab in previously infliximab-treated patients with inflammatory bowel disease. Dig Dis Sci 2018; 63: 1583–1591. https://doi.org/10.1007/s10620-018-5020-9
24. Karmiris K, Paintaud G, Noman M, Magdelaine-Beuzelin C, Ferrante M, Degenne D et al. Influence of Trough Serum Levels and Immunogenicity on Long-term Outcome of Adalimumab Therapy in Crohn’s Disease. Gastroenterology 2009; 137: 1628–1640. https://doi.org/10.1053/j.gastro.2009.07.062
25. Matsumoto T, Motoya S, Watanabe K, Hisamatsu T, Nakase H, Yoshimura N et al. Adalimumab Monotherapy and a Combination with Azathioprine for Crohn’s Disease: A Prospective, Randomized Trial. J Crohns Colitis 2016; 10: 1259–1266. https://doi.org/10.1093/ecco-jcc/jjw152
