Eredeti közlemény / Original paper
IBD-related Malignancies Observed in 2015–2019
Summary
Introduction: Patients with inflammatory bowel disease (IBD) have an increased risk to develop malignant neoplasms. Neither the exact mechanism, nor the frequency of different malignancies is completely clear. Our aim was to assess the IBD associated malignancies, to collect clinical and mortality data and to analyse possible risk factors. Methods: Data on malignancies developed between January 2015 and May 2019 in Hungarian IBD patients was recorded. Every member of the Hungarian Society of Gastroenterology was prospectively interviewed. The following data were collected: demographic data, disease characteristics, previous therapy, patient adherence, type and localisation of malignancies. Results: 140 IBD patients with newly diagnosed malignancies were reported. 61.4%, 35.7%, and 2.9% of the patients had ulcerative colitis (UC), Crohn’s disease (CD) and indeterminate colitis, respectively. The mean latency was 15.2±10.5 years. Colorectal cancer (CRC) was the most common cancer (49.6%, 70). 72.9% (51/70) of them was associated with UC, more than 80% had extensive (50.9%, 26) and left-sided (31.2%, 16) colitis. The most frequent CRC localisation was the rectosigmoid colon in UC (54.9%), and the rectum in CD (38.9%). The most common non-CRC malignancies were non-melanotic skin-cancer, haematological and pulmonary cancer. Disease duration at the time of the diagnosis of malignancy was lower (17.9±10.7 versus 12.6±9.7 years); mean age at the time of the death was higher (49.3±9.4 versus 64.3±16.4 years); and survival was longer after the diagnosis of extraintestinal malignancy than CRC (0.73±1.01 versus 1.2±0.8). Summary: CRC presented typically in the distal part of the colon by male UC patients with pancolitis or left-sided colitis with a long-standing disease course of IBD. The most common non-CRC malignancies were non-melanotic skin cancer, haematological cancer and lung cancer. Non-CRC malignancies developed typically in female patients, older than CRC patients with shorter disease-course of IBD and longer survival times.
Bevezetés: A gyulladásos bélbetegségben (IBD) szenvedő betegeknél fokozott a rosszindulatú daganatok kialakulásának kockázata. Sem a pontos mechanizmus, sem a különböző malignitások gyakorisága nem teljesen tisztázott. Célunk az IBD-vel összefüggő rosszindulatú daganatok felmérése, klinikai és halálozási adatok gyűjtése, valamint a lehetséges kockázati tényezők elemzése volt.
Módszerek: A 2015 januárja és 2019 májusa között, magyar IBD-s betegeknél kialakult rosszindulatú daganatok adatait rögzítettük. A Magyar Gasztroenterológiai Társaság minden tagját prospektív módon kérdeztük. A következő adatokat gyűjtöttük: demográfiai adatok, betegségjellemzők, korábbi terápia, betegadherencia, a malignitások típusa és lokalizációja.
Eredmények: 140 IBD-s betegről számoltak be, akiknél újonnan diagnosztizált rosszindulatú daganatot állapítottak meg. A betegek 61,4%-a colitis ulcerosában (UC), 35,7%-a Crohn-betegségben (CD), 2,9%-a pedig indeterminált colitisben szenvedett. Az átlagos latenciaidő 15,2±10,5 év volt. A vastagbélrák (CRC) volt a leggyakoribb daganatos megbetegedés (49,6%, 70). Ezek 72,9%-a (51/70) UC-vel társult, több mint 80%-uknak kiterjedt (50,9%, 26) és bal oldali (31,2%, 16) colitise volt. A leggyakoribb CRC-lokalizáció UC-ben a rectosigmoid colon (54,9%), CD-ben a végbél (38,9%) volt.
A leggyakoribb nem-CRC rosszindulatú daganatok a nem melanotikus bőrrák, hematológiai daganatok és a tüdőrák voltak. Az extraintesztinális daganatok megjelenésénél a betegség átlagos fennállása rövidebb volt (17,9±10,7 versus 12,6±9,7 év), az átlagos életkor a halál idejében magasabb volt (49,3±9,4 versus 64,3±16,4 év), és a túlélés hosszabb volt (0,73±1,01 versus 1,2±0,8 év) mint colorectalis rák esetében.
Összefoglalva: A CRC jellemzően a vastagbél disztális részében jelent meg colitis ulcerosás férfi betegeknél, pancolitis vagy bal oldali colitis ulcerosa esetén, hosszabb betegségfennállás után. A leggyakoribb nem CRC rosszindulatú daganatok a nem melanotikus bőrrák, hematológiai daganatok és a tüdőrák voltak. A nem CRC daganatok gyakoribbak voltak a nőbetegeknél, idősebb életkorban jelentkeztek, mint a CRC, rövidebb betegségfennállás után, és hosszabb túlélés jellemezte.
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are lifelong diseases with a chronic inflammation of the gastrointestinal (GI) tract. Patients with inflammatory bowel disease (IBD) have an increased risk to develop malignant neoplasms, particularly colorectal cancer (CRC) in ulcerative colitis and colon Crohn’s disease because of the chronic intestinal inflammation, however recent studies suggest the rate is decreasing thanks to improved surveillance (1–7). Colorectal cancer is the third most common cancer worldwide and accounts for 10-15% of all deaths in IBD (8). IBD specific risk factors for CRC are ulcerative colitis and colon Crohn’s disease, longer duration of IBD (8 years or more after diagnosis), extensive colon inflammation and coexisting primary sclerosing cholangitis (PSC), while male gender, increasing age and positive family history of CRC are risk factors to sporadic CRCs (9–11). Recently published data suggest that the risk of extraintestinal malignancies is also increased in IBD patients (12, 13). Disease-, patients- and therapy-related causes are taken into consideration; however, neither the exact mechanism, nor the frequencies of different malignancies are completely clear. CD patients are more likely to develop extraintestinal malignancies than UC patients. Upper GI cancer, lung cancer, urinary bladder cancer, skin squamous cell cancer, breast cancer were more frequent among CD patients and liver-biliary cancer, leukaemia, breast cancer and corpus uteri cancer among UC patients (5, 13, 14).
Due to the chronic nature of IBD, the impact of frequently used therapy in cancer development is still the subject of intensive debate. Thiopurine use can increase the risk of cancer, especially for lymphoma, haematologic cancer, skin squamous cell cancer (15–17). The currently available meta-analyses suggest that the overall risk of cancer is not increased in patients treated with anti-TNF-alpha therapy alone (18, 19).
Our aim in this prospective, nation-wide study was to assess the IBD associated malignancies, to collect clinical and mortality data and to analyse possible risk factors.
Methods
Data on malignancies developed between January 2015 and May 2019 in Hungarian IBD patients was recorded. Every member of the Hungarian Society of Gastroenterology was prospectively interviewed every 6 months by personal e-mail to report malignancies observed in their patient population. The following data was collected in Excel-spreadsheets: IBD-related data: demographic data including gender, age at the time of the diagnosis of IBD, disease characteristics, such as type and nature of IBD, previous immunosuppressive or biological therapy, patient adherence; malignancy related data: age at the time of the diagnosis of malignancy, localisation, TNM stage of the tumour and in case of colorectal cancer the year of the previous colonoscopy. In the end of May 2019 all physicians were interviewed about the previously reported patients’ status. Patients were eligible for the study if they had inflammatory bowel disease before or at the time of the diagnosis of malignant cancer. All type of malignant cancers was taken into account. The stage of colorectal cancer was based on the 8th Edition TNM Staging system.
A descriptive statistical analysis was performed. Statistical data was reported as the mean ± SD (standard deviaton); with frequencies (n) and percentages (%), when appropriate, for the survival time mean ± SE (standard error) were calculated. Pearson’s chi-squared test or Fisher’s exact test was used to analyse categorical data, whereas independent samples t-test was used in case of continuous data. The survival probabilities were estimated by Kaplan-Meier analysis. The differences in the survival of the CRC and non-CRC groups were examined by a log-rank test. Statistical tests were performed using R statistical software (R version 3.6.2, https://www.r-project.org/), values of P<0.05 were considered significant.
The study was approved by the Ethical Committee (3929, 15/2017-SZTE). The data are collected and analysed anonymously.
Results
Demographic data
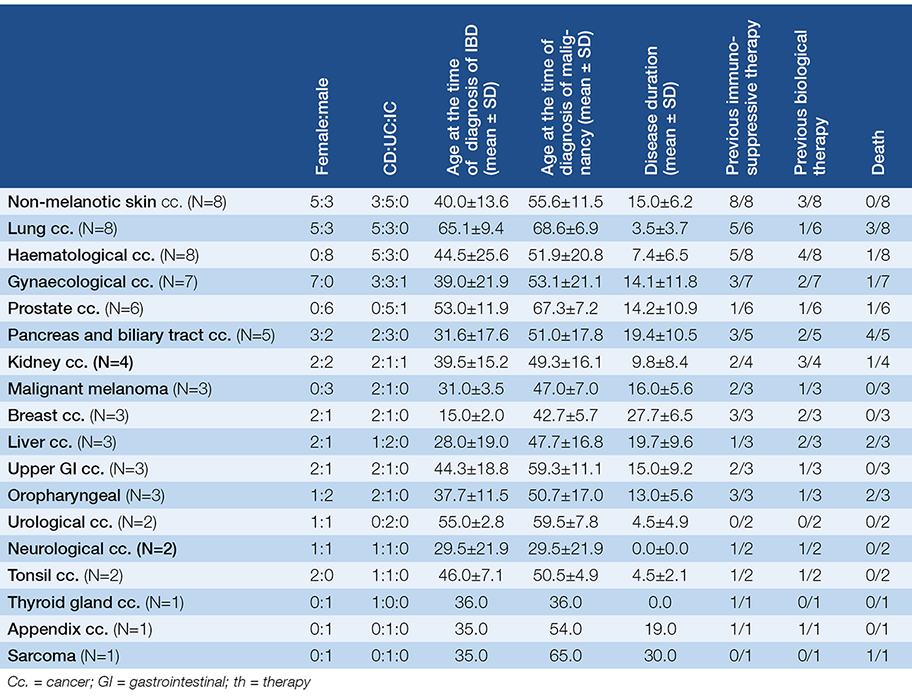
140 IBD patients were reported by the members of the HSG. Patients were reported from 20 departments, altogether from 11 cities (Békéscsaba, Budapest, Debrecen, Gyula, Nyíregyháza, Pápa, Pécs, Szeged, Szekszárd, Szombathely, Veszprém). The majority of patients were male (female:male ratio was 35:65%; 49:91 patients). 61.4%, 35.7%, and 2.9% of the patients had UC, CD and indeterminate colitis (IC), respectively. The mean age of the patients at the time of initial diagnosis of malignancy was 53.0±14.7 (range 14-92) years. At the time of initial diagnosis of IBD, the mean age was 38.0±18.0 (range 8-86) years. The mean latency between the diagnosis of IBD and malignancy was 15.2±10.5 (range 0-52) years. Altogether 141 malignancies were reported – one patient had simultaneously two different types of tumour (descending colon and kidney), we counted it as two cases in the subgroup analyses.
Occurrence of malignancies
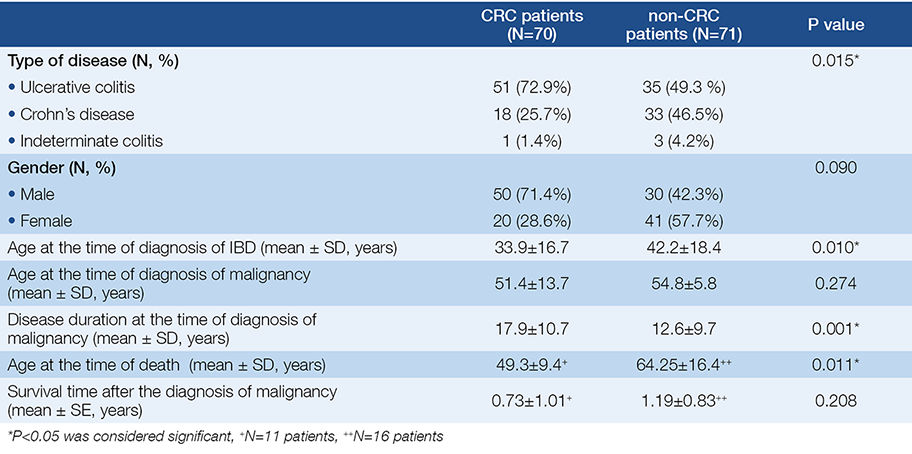
According to our results CRC was the most common cancer type (49.6%, 70), while the most common non-CRC malignancies were non-melanotic skin-cancer (5.7%, 8), haematological and pulmonary cancer (5.7-5.7%, 8-8) (Fig. 1.). The proportion of UC patients is significantly higher in the CRC subgroup contrary to the non-CRC subgroup (P=0.015). CRC patients were significantly younger at the time of diagnosis of IBD (P=0.01), had a longer disease duration before the diagnosis of malignancy (P=0.001) and died younger (P=0.011) contrary to non-CRC patients. There was no statistical difference in gender and the age at the time of diagnosis of malignancy. Table 1. shows the demographic data of the CRC and non-CRC patients in detail.
Fig. 1. Distribution of malignancies in inflammatory bowel disease
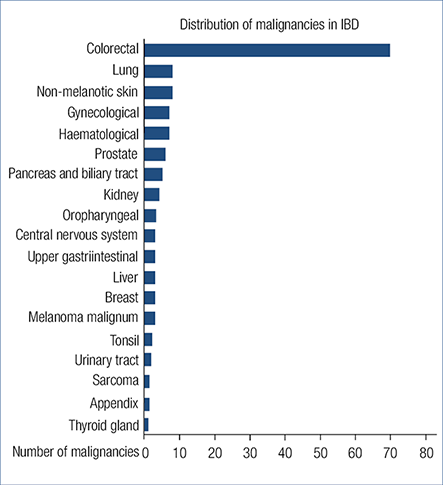
Table 1. Demographic data of patients suffering from colorectal cancer (CRC) and non-colorectal cancer (non-CRC)
Colorectal cancer
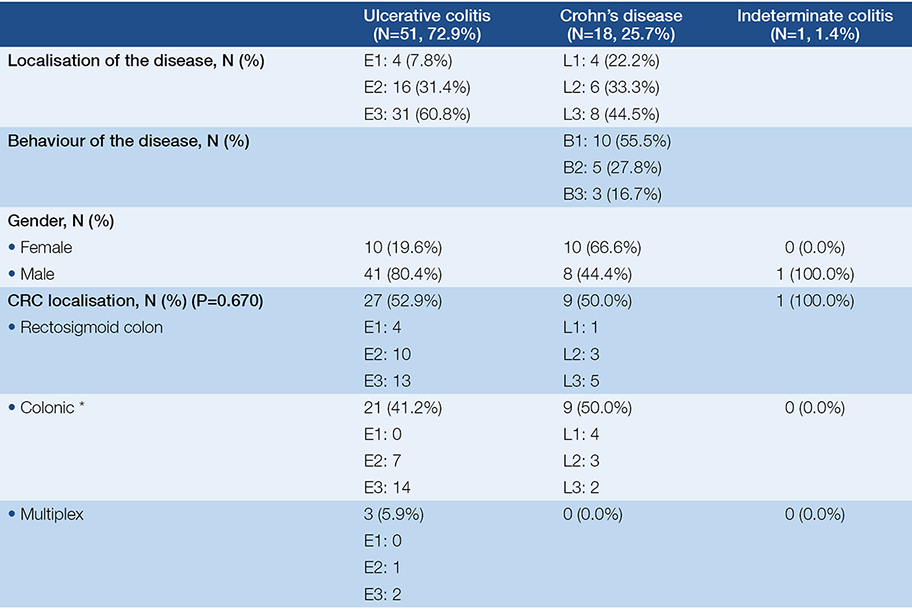
As CRC can be a result of long-term IBD, it was analysed in detail. 70 patients were diagnosed with CRC. 72.9% (51 of 70) of CRC cases was associated with ulcerative colitis, more than 80% of them had extensive (50.9%, 26) and left-sided (31.2%, 16) colitis. Among CD patients ileocolonic and colonic localisation was observed in 44.4% (8/18) and 33.3% (6/18). In UC patients a male dominance was seen (80.4%). The mean age at the time of diagnosis of malignancy was 51.4±13.7 years. Mean disease duration of IBD was 17.9±10.7 years, but it was less than 8 years in 10 patients (70% of them had early stage CRC). In 5 cases CRC diagnosis was simultaneous with the diagnosis of IBD. Demographic data is detailed in Table 2. 27 patients (38.6%) had previous colonoscopies before the diagnosis of CRC, a mean 3.85±3.2 years. 21.4% (15 patients) of the patients were non-adherent to regular check-ups. Family history was positive to CRC in 15.7% (11 patients).
Table 2. Demographic data of inflammatory bowel disease patients suffering from colorectal cancer. *(descending, transverse, ascending colon and coecum)
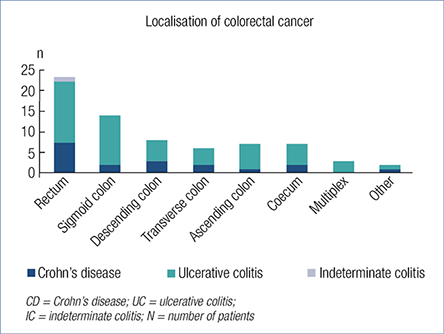
The most common CRC localisation in UC patients was the rectosigmoid colon (52.9%, 27), and the rectum in CD patients (38.9%, 7) (Figure 2.). There was no statistical difference between the tumour localisation and the nature of the disease. Three patients had multiplex tumours: one ulcerative colitis (E2) patient had simultaneous adenocarcinoma in coecum and rectum; the second ulcerative colitis patient (E3) had three adenocarcinomas in coecum, descending colon and rectum; the third ulcerative colitis patient (E3), had four adenocarcinomas in colon and one in rectum. Tumour localisation of UC and CD patients is detailed in Fig. 2. Two patients had IBD-related operations previously where the malignancy developed: one UC patient had pouch adenocarcinoma, the other patient with Crohn’s disease had adenocarcinoma in the ileotransverse anastomosis.
Fig. 2. Localisations of colorectal cancer in inflammatory bowel disease
The nature of the disease was also evaluated. Chronic continuous symptoms, chronic remission, relapse and remission, relapse after chronic remission was observed in 37.1% (26), 24.3% (17), 20.0% (14) and 2.9% (2) of the patients, respectively (15.7%, 11 patients had no data available). Half of the patients with CD had non-stricturing, non-penetrating disease, 27.8% had stenotisating and 16.7% had penetrating disease behaviour (5.5% had no data about the behaviour of the disease).
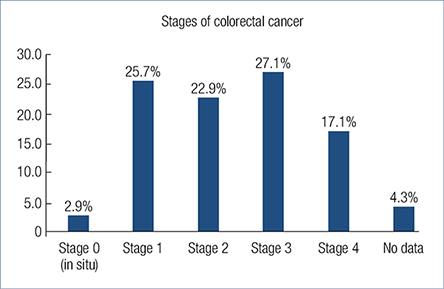
Only 28.7% (20 patients) of the malignancies were detected in early stage (Stage 0 or Stage 1). One (5%) of them died in pneumonia and sepsis in the year of the tumour diagnosis. Stage 2, Stage 3 and Stage 4 tumour was detected in 50 patients (71.3%), 10 (18%) of them died after a mean 0.55 year (Fig. 3.). There was no statistical difference in the previously used therapy (immunosuppressive, biological therapy or both) and the stage of the CRC.
Fig. 3. Stages of colorectal cancer at the time of the diagnosis of malignancy
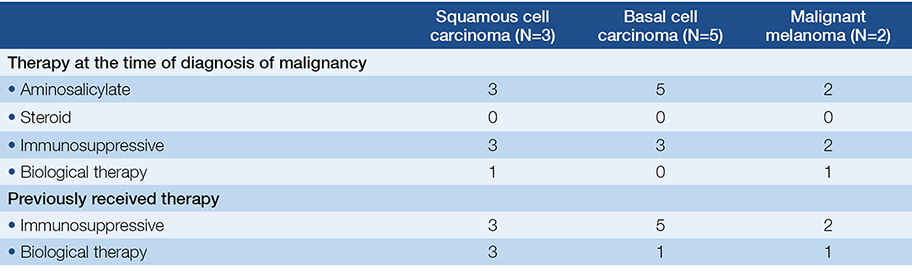
At the time of the diagnosis of malignancy 71.4% (50) of the patients received aminosalicylates, 37.1% (26), 28.6% (20) received steroid and/or immunosuppressive therapy, and only 11.4% (8) received biological therapy. Previously 42.9% (30) of the patients received immunosuppressive therapy a mean 63.3±58.1 months, 60% of them received azathioprine (AZA) alone, and 40% received a combination therapy.
Non-colorectal cancers
The most frequently observed non-CRC malignancies in our cohort were non-melanotic skin-cancer (5.7%, 8), haematological (5.7%, 8) and pulmonary cancer (5.7%, 8), then gynaecological and prostate malignancies in 5% and 4.3% (7 and 6 patients). Non-colorectal cancers are detailed in Table 3.
Table 3. Summary of non-colorectal cancers
Non-melanotic skin cancers (NMSC) were the following: 5 cases had basal cell carcinoma and 3 cases had squamous cell carcinoma. All of the patients with NMSC had previous immunosuppressive therapy for a mean 79.8±50.8 (12-144) months. IBD-related therapy of patients with skin cancer is detailed in Table 4.
Table 4. IBD-related therapy of patients with skin cancer
8 patients had haematological malignancies, all of them were male. Three of them had ulcerative colitis with the following leukaemia: chronic myeloid leukaemia, acute
myeloid leukaemia and chronic lymphoid leukaemia, and five of them had Crohn’s disease with the following diseases: diffuse large B-cell lymphoma (2 cases), non-
Hodgkin lymphoma, Waldenström macroglobulinemia and chronic myeloid leukaemia.
8 patients had lung cancer, 5 patients had Crohn’s disease, 3 had ulcerative colitis. 7 patients had gynaecological malignancy. 6 patients had a prostate tumour, only one of them had long-term combination immunosuppressive treatment. Mean age at the time of diagnosis of prostate malignancy was 67.3 years (min: 54, max: 74); the 54 years old patient was that who had long term combination immunosuppressive treatment, all the other patients were over 65 years old.
IBD-related medical therapy was the following in the non-CRC subgroup: 76.1% (54) of the patients were on aminosalicylates, 15.5% (11) on steroids, 38.0% (27) on immunosuppressive therapy and 18.3% (13) on biological therapy at the time of the diagnosis of malignancy. Previously, 56.3% (40) received azathioprine (68.5±56.9 years): 23.9% (17) received azathioprine alone, 32.4% (23) received combination therapy and 5.6% (4) received biological therapy alone.
Comorbidities
Primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC) was analysed in detail. 10 cases were associated with PSC, from them 6 had CRC, 2 had liver malignancy and 2 patients had biliary adenocarcinoma. 2 patients with colorectal cancer had PBC. Mean age at the time of diagnosis of CRC in patients with PSC or PBC was lower contrary to patients without comorbidities (45.5±7.4 versus 52.2±14.2 years).
Survival
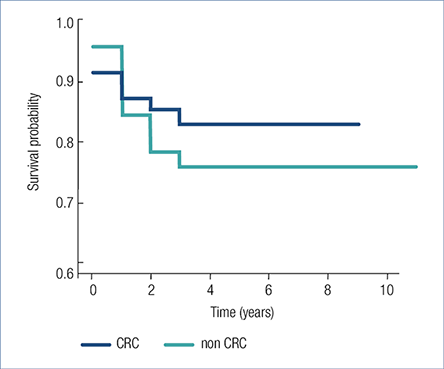
19.3% (27/140) patients died after the diagnosis of malignancy after a mean 1.0±0.92 years, 86% of the patients were adherent to regular check-ups. 15.7% (11 of 70) of CRC patients and 22.5% (16 of 71) of non-CRC patients died during the follow-up period. Male dominancy was observed (19:8). CRC patients died significantly younger than non-CRC patients (49.3±9.4 versus 64.3±16.4 years). There was no statistical difference between the survival of CRC (8.44±0.434 years) and non-CRC (7.91±0.459 years) patients (P=0.458) (Fig. 4.) and the survival of early or late stage colorectal cancers (P=0.758).
Fig. 4. Survival after the diagnosis of colorectal cancer and non-colorectal cancer
Discussion
In our nationwide prospective study 140 IBD patients were reported with malignancy over a time course of 4 years (2015–2019).
Based on our findings the most common malignancies were colorectal cancers (49.6%), non-melanotic skin cancers (5.8%), haematological neoplasms (5.8%) and malignant neoplasms of the lung (5.8%). These data are in concordance with the recently published Hungarian population-based data, where 8.5% of the incident UC population was diagnosed with CRC (20). Similar data were presented by a Danish population-based study from 1962 to 1987, where increased risk of colorectal cancer and melanoma was found in UC patients, but for other cancers they couldn’t verify this (21). A Scandinavian population based-study also found an increased risk of developing CRC both in UC and CD patients (HR [hazard ratio]: 1.66 and 1.40) (2, 3). Other studies suggest that the risk of CRC is declining over time (1, 22, 23). A recently published Swiss IBD cohort study found controversial results concerning CRC: they found an increased risk for lymphoma and biliary cancer, but not for colorectal cancer (22).
Colorectal cancers were more frequent in UC patients and developed after a significantly longer disease duration contrary to non-CRC patients (17.9 versus 12.6 years). In UC patients with colorectal cancer a male dominance was seen. Non-CRC patients were older at the time of death (64.3 versus 49.3 years); and survival time was longer (1.2 versus 0.73 years) after the diagnosis of non-CRC malignancy than CRC. These findings are in concordance with the previously published findings, which showed that ulcerative colitis increases the risk of CRC and male gender, younger age at diagnosis of UC (disease duration), extensive colitis and geography were independent risk factors (9, 24).
Based on our results the most common CRC localisation in UC patients was the rectosigmoid part of the colon (54.9%), and the rectum in CD patients (38.9%). In line with our data, increased risk for CRC and hepatobiliary cancers among IBD patients was found, and rectal cancer was more frequent among UC patients (25), but not among CD patients (6). 85.7% of the patients had long-standing disease (17.9 years) in our cohort, it should be noted that it was less than 8 years in 10 patients (70% of them had early stage CRC), which can be a result of diagnostic delay. Primary sclerosing cholangitis can increase the risk of CRC in IBD patients, especially in ulcerative colitis patients (26). In our cohort IBD patients with PSC were younger at the time of CRC onset, contrary to patients with IBD alone.
Colorectal cancer stages in our cohort are in concordance with the findings of the Hungarian population-based colorectal cancer screening pilot study’s result (27). In this pilot study stage 0 and 1 tumour was diagnosed in 26.7%, stage 2 in 27.2%, stage 3 in 22.6% and stage 4 in 23.5%, while in our cohort the data was 28.6%, 22.9%, 27.1% and 17.1%, respectively.
Non-CRC patients were predominantly female. The most common non-CRC malignancies were non-melanotic skin cancers, haematological neoplasms and malignant neoplasms of the lung. In our cohort all the patients with leukaemia suffered from UC. Although the overall risk of extra-intestinal malignancies is not increased in IBD, the individual cancer types depending on the background population, CD patients have an especially increased risk to develop cancers (10, 13, 28). Contrary to our results, some studies found increased risk of lymphoma in CD patients, especially in males. This risk was independent of thiopurine use (6, 25, 28). Pedersen et al found an increased risk of cancer of the upper gastrointestinal tract (SIR [standardised incidence ratio]: 2.85), lung (SIR: 1.82), urinary bladder (SIR: 2.03) and skin (SIR: 2.35) among CD patients and of leukaemia (SIR: 2.0) and liver-biliary cancer (SIR: 2.58) among UC patients. A Spanish retrospective, multi-centre, 5-year follow up, observational study found an increased risk for non-melanoma skin-cancer and for small-bowel cancer (RR [relative risk] = 3.85 and 3.70) (23). This difference in the distribution of extraintestinal malignancies may be a result of different smoking habits and extra-intestinal manifestations (13).
All the patients with non-melanotic skin cancer was on longstanding immunosuppressive therapy for a mean 79.8 months. All the patients had squamous cell carcinoma and basal cell carcinoma, which is in range with previous study’s results (13). Patients on thiopurine therapy has increased risk of non-melanoma skin cancer (12), haematologic cancer, non-Hodgkin lymphoma, skin squamous cell carcinoma and overall cancer (17).
This study has some limitations. However, this study was a prospective study there is still some incomplete data (medication, cancer stage, adherence). It could happen because not all the patients were initially diagnosed with IBD in the referral centre/physician, and some patients haven’t returned to the referral physician during the study period. This database is not representative of the Hungarian population, since participation was voluntary. The CRC cases can be overestimated, due to the fact that patients with CRC visit gastroenterological centres more frequently, while patients with extraintestinal malignancies may be out of sight after the diagnosis of malignancy for years.
Conclusion
The most frequently observed IBD-related malignancy in our cohort was colorectal cancer. Colorectal cancer presented typically in the distal part of the colon by male ulcerative colitis patients with pancolitis or left-sided colitis with a long-standing disease course of IBD. The most common non-colorectal cancer malignancies were non-melanotic skin cancer, haematological cancer and lung cancer. Patients with non-colorectal cancer malignancies were typically female and older than colorectal cancer patients at the time of the diagnosis of malignancy had a shorter disease-course of IBD and a longer survival time.
Acknowledgments
We thank the participants of the Hungarian IBD Study Group – Ágota Kovács, Patrícia Sarlós, Márta Varga, András Gelley, Zsuzsanna Kürti, Péter Lakatos, Lilla Lakner, Károly Palatka, Tamás Szamosi, Áron Vincze, Mihály Balog, Zsolt Barta, Ákos Iliás, János Novák, Tünde Pandur, Nóra Szigeti, János Banai, Ildikó Kovács, Márta Kovács, Pál Miheller, Ágnes Salamon, Valéria Sipos, Gyula Tolvaj, Gábor Veres, Tibor Wittmann, Alexandra Zádori-Born, Edit Balla, Anita Gaál, Krisztián Pepa – for their active participation in the study.
https://doi.org/10.1093/ecco-jcc/jjz058.
2. Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, Ekbom A, Sørensen HT, Ludvigsson JF. Colorectal cancer in Crohn’s disease: a Scandinavian population-based cohort study. Lancet Gastroenterol Hepatol. 2020 May 1; 5(5): 475–484.
https://doi.org/10.1016/S2468-1253(20)30005-4.
3. Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, Ekbom A, Sørensen HT, Ludvigsson JF. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020 Jan 11; 395(10218): 123–131.
https://doi.org/10.1016/S0140-6736(19)32545-0.
4. Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013; 19(4): 789–799.
https://doi.org/10.1097/MIB.0b013e31828029c0.
5. Taborelli M, Sozzi M, Del Zotto S, Toffolutti F, Montico M, Zanier L, Serraino D. Risk of intestinal and extra-intestinal cancers in patients with inflammatory bowel diseases: A population-based cohort study in northeastern Italy. PLoS One. 2020 Jun 23; 15(6): e0235142.
https://doi.org/10.1371/journal.pone.0235142
6. von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The Risk of Cancer in Patients with Crohn’s Disease. Dis Colon Rectum. 2007 Jun; 50(6): 839–855.
https://doi.org/10.1007/s10350-006-0848-z.
7. Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-Year Analysis of a Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis. Gastroenterology. 2006; 130(4): 1030–1038.
https://doi.org/10.1053/j.gastro.2005.12.035
8. Herszényi L, Barabás L, Miheller P, Tulassay Z. Colorectal Cancer in Patients with Inflammatory Bowel Disease: The True Impact of the Risk. Dig Dis. 2015; 33(1): 52–57. https://doi.org/10.1159/000368447.
9. tients With Ulcerative Colitis: A Meta-analysis of Population-Based Cohort Studies. Clin Gastroenterol Hepatol. 2012; 10(6): 639–645.
https://doi.org/10.1016/j.cgh.2012.01.010.
10. Annese V, Beaugerie L, Egan L, Biancone L, Bolling C, Brandts C, Dierickx D, Dummer R, Fiorino G, Gornet JM, Higgins P, Katsanos KH, Nissen L, Pellino G, Rogler G, Scaldaferri F, Szymanska E, Eliakim R; ECCO. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. Journal of Crohn’s and Colitis. 2015 Nov; 9(11): 945–965. https://doi.org/10.1093/ecco-jcc/jjv141.
11. Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. Vol. 372, New England Journal of Medicine. 2015; 372: 1441–1452. https://doi.org/10.1056/NEJMra1403718
12. Chang M, Chang L, Chang HM, Chang F. Intestinal and Extraintestinal Cancers Associated With Inflammatory Bowel Disease. Clinical Colorectal Cancer. 2018; 17(1): e29–37.
https://doi.org/10.1016/j.clcc.2017.06.009
13. Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: Meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010 Jul; 105(7): 1480–1487. https://doi.org/10.1038/ajg.2009.760.
14. Hovde Ø, Høivik ML, Henriksen M, Solberg IC, Småstuen MC, Moum BA. Malignancies in patients with inflammatory bowel disease: Results from 20 years of follow-up in the IBSEN study. J Crohns Colitis. 2017; 11(5): 571–577. https://doi.org/10.1093/ecco-jcc/jjw193.
15. Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: A UK population-based case-control study. Am J Gastroenterol. 2010 Jul; 105(7): 1604–1609. https://doi.org/10.1038/ajg.2009.745.
16. Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013 Jun 1; 177(11): 1296–1305.
https://doi.org/10.1093/aje/kws375.
17. van den Heuvel TRA, Wintjens DSJ, Jeuring SFG, Wassink MHH, Romberg-Camps MJL, Oostenbrug LE, Sanduleanu S, Hameeteman WH, Zeegers MP, Masclee AA, Jonkers DM, Pierik MJ. Inflammatory bowel disease, cancer and medication: Cancer risk in the Dutch population-based IBDSL cohort. Int J Cancer. 2016 Sep 15; 139(6): 1270–1280. https://doi.org/10.1002/ijc.30183.
18. Williams CJM, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: Malignancies with anti-tumour necrosis factor-a therapy in inflammatory bowel disease. Vol. 39, Aliment Pharmacol Ther. 2014. Mar; 39(5): 447–458. https://doi.org/10.1111/apt.12624.
The rest of the references can be found in the editorial office and on the website http://www.gastronews.hu.19. Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Bonovas S. Systematic review with meta-analysis: biologics and risk of infection or cancer in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2020 May; 51(9): 820–830. https://doi.org/10.1111/apt.15692.
20. Kunovszki P, Milassin Á, Gimesi-Országh J, Takács P, Szántó K, Bálint A, Farkas K, Borsi A, Lakatos PL, Szamosi T, Molnár T. Epidemiology, mortality and prevalence of colorectal cancer in ulcerative colitis patients between 2010-2016 in Hungary – a population-based study. PLoS ONE. 2020 May 14; 15(5): e0233238. https://doi.org/10.1371/journal.pone.0233238.
21. Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: A population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004 Dec; 2(12): 1088–1095. https://doi.org/10.1016/s1542-3565(04)00543-9.
22. Scharl S, Barthel C, Rossel JB, Biedermann L, Misselwitz B, Schoepfer AM, Straumann A, Vavricka SR, Rogler G, Scharl M, Greuter T. Malignancies in Inflammatory Bowel Disease: Frequency, Incidence and Risk Factors – Results from the Swiss IBD Cohort Study. Am J Gastroenterol. 2019 Jan 1; 114(1): 116–126. https://doi.org/10.1038/s41395-018-0360-9.
23. Algaba A, Guerra I, Marín-Jiménez I, Quintanilla E, López-Serrano P, García-Sánchez MC, Casis B, Taxonera C, Moral I, Chaparro M, Martín-Rodríguez D, Martín-Arranz MD, Manceñido N, Menchén L, López-Sanromán A, Castaño Á, Bermejo F. Incidence, management, and course of cancer in patients with inflammatory bowel disease. J Crohns Colitis. 2015; 9(4): 326–333. https://doi.org/10.1093/ecco-jcc/jjv032.
24. Zhou Q, Shen ZF, Wu BS, Xu CB, He ZQ, Chen T, Shang HT, Xie CF, Huang SY, Chen YG, Chen HB, Han ST. Risk of Colorectal Cancer in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2019; 2019: 5363261. https://doi.org/10.1155/2019/5363261.
25. Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer Risk in Patients with Inflammatory Bowel Disease A Population-Based Study. Cancer. 2001 Feb; 91(4): 854–862.
https://doi.org/10.1002/1097-0142(20010215)91:4<854::aid-cncr1073> 3.0.co;2-z.
26. Wang R, Leong RW. Primary sclerosing cholangitis as an independent risk factor for colorectal cancer in the context of inflammatory bowel disease: A review of the literature. World J Gastroenterol. 2014. Jul; 20(27): 8783–8789. https://doi.org/10.3748/wjg.v20.i27.8783.
27. Rutka M, Bor R, Molnár T, Farkas K, Fábián A, Györffy M, Bálint A, Milassin Á, Szücs M, Tiszlavicz L, Nagy F, Szepes Z. Efficacy of a population-based colorectal cancer screening pilot. Turkish J Med Sci. 2020 Mar 10; https://doi.org/10.3906/sag-1908-79.
28. Jess T, Horváth-Puhó E, Fallingborg J, Rasmussen HH, Jacobsen BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: A danish population-based cohort study. Am J Gastroenterol. 2013 Dec; 108(12): 1869–1876. https://doi.org/10.1038/ajg.2013.249.
