Clinical and endoscopic scores in ulcerative colitis
Summary
Ulcerative colitis is an immune-mediated bowel disease of unknown etiology, which requires long-term treatment and follow-up. The disease is characterized by relapses, and clinical and endoscopic indices can be of great tool in monitoring it, allowing easier comparability, objective, clear documentation and therapeutic decisions. Many clinical and endoscopic score systems have been developed, but only a fraction of them are validated, however, simplicity of use has a priority in practice.
A colitis ulcerosa ismeretlen etiológiájú, immun-mediált bélbetegség, amely élethosszig tartó kezelést és utánkövetést igényel. A betegség fellángolásokkal tarkított lefolyást mutat, amelynek monitorozásában nagy segítséget nyújthatnak a klinikai és endoszkópos indexek, amelyek könnyebb összehasonlíthatóságot, objektivizálást tesznek lehetővé az egyértelmű dokumentáció és a terápiás döntések elősegítése mellett. Számos klinikai és endoszkópos pontrendszer áll rendelkezésünkre, amelyeknek csupán töredéke validált, azonban a gyakorlatban inkább az egyszerűség és a könnyű alkalmazhatóság jelent prioritást.
Introduction
Ulcerative colitis (UC) is a chronic, relapsing immune-mediated bowel disease with the need of precise patient follow-up. The significance of diagnosis and management of UC lied in the severity of disease outcomes (like toxic megacolon, need of colectomy) without appropriate therapy. Indices provide information about disease activity at a given time. During evaluation of severity and outcome of UC, the relevant issues are clinical symptoms, quality of life and disability, disease extent and course including structural damage (1).
Clinical indices provide a noninvasive method for clinical disease assessment, facilitating the everyday management of UC by allowing for easier comparability, objective, clear documentation and therapeutic decisions. Nowadays, medical treatment of UC can induce and maintain clinical remission in most of the cases moreover most of the studies suggest a new endpoint of therapy that has been associated with better disease outcome: the mucosal healing. Mucosal healing is associated with decreased need for corticosteroids, decreased hospitalisation, colectomy and bowel resection rates, sustained clinical remission, and decreased risk for colorectal cancer (2). Definition of mucosal healing is quite complex, and it is more than the absence of ulcerations. An International Organisation of Inflammatory Bowel Disease define it in UC as the absence of friability, blood, erosions and ulcers in all visualized segments of the colonic mucosa (2).
Statement of mucosal healing is based on interpretation of the endoscopic features. To note, although colon mucosa may seem to be intact, microscopic inflammation may persist. Nevertheless, preferring the endoscopic score systems in everyday practice can reduce the subjective factors. There are many indices in UC, however none of them is perfect; some scores are developed from another previously used index, thus it has adopted the same or slightly the same parameters, but ignore symptoms important to patients (urgency, fecal incontinence etc.). Standard definitions have not been defined for most of signs and symptoms important to physicians and patients. For example, it is hard to define bowel movements for patients with fecal incontinence due to severe disease activity. To note, only a few have formal validation, like UCEIS, UCCIS, but not Mayo Score which is the most popular index among clinicians (3).

The clinical scores in ulcerative colitis
Grading clinical disease activity into mild, moderate and severe categories is based on signs and symptoms of UC. Severe UC was described originally by Trulove and Witts in 1955 with criteria of 6 or more bloody bowel movements per day, fever, tachycardia, anemia, ESR >30 mm/h and need for hospitalization (Truelove and Witts Severity Index) (4). In this study, oral cortisone treatment efficacy was investigated in UC patients, and they used threshold terms „clinical remission”, „improvement”, „no change” or „worsening” that are not quantitative. Truelove and Witts criteria is useful for recognize severe UC, however it is not sufficiently discriminative to measure fine distinction of disease activity. Nonetheless, Dinesen et al. confirmed strong association between severe UC defined by Truelove and Witts criteria and rate of colectomy (5).
In 1978, Powell–Tuck et. al developed an index concerned to investigation about comparison of oral prednisolone 10 mg 4 times daily vs. 40 mg once daily for treatment of active UC. The Powell–Tuck Index include 10 descriptive (general health, abdominal pain, bowel movement frequency, stool consistency, bleeding, anorexia, nausea or vomiting, abdominal tenderness, extraintestinal manifestations and temperature) and score ranging from 0 to 20 points (6). Variation of this score include sigmoideoscopic findings (+0-2 points, total 0–22). The study that assessed coated oral 5-aminosalicylic acid therapy for mild to moderate active UC by Schroedeer et al. has a great significance not just because they found 5-ASA an effective treatment, but they developed a score system – the Mayo Score – which is the most widely used score nowadays (7). In 1989, Rachmilewitz et al. reported from the study coated mesalamine vs. sulphasalazine a clinical activity index and an endoscopic score, as well. Clinical Activity Index can be calculated by stool frequency, bleeding, investigator’s global assessment, abdominal pain, temperature, extraintestinal manifestations and laboratory findings. It has been validated in a study which define clinical remission as CAI ≤4 points (8), Lichtinger et al. described a modified Truelove and Witts Severity Index in a clinical trial in 1990. There are 8 parameters included: number of daily stools, nocturnal stools, visible blood in the stool, fecal incontinence, abdominal pain, general well-being, abdominal tenderness and need for antidiarrheal. The index ranges in 0–21 points the remission is ≤3 points (8).
In 1993, Hanauer et al. utilized a Physician Global Assessment (PGA) in a placebo-controlled trial of sustained released mesalazine. Levine et al developed Improvement Based On Individual Symptom Scores in 2002, than Feagan et al reported results from a study which used a modification of Mayo Score, named the Ulcerative Colitis Clinical Score. This clinical score consists of variables as rectal bleeding, stool frequency, functional assessment by the patient and global assessment by the physician. In the same study the Modified Baron Score was used to assess endoscopic features (9). In the era of biologicals, scoring systems became more and more significant tool of determination of response to therapy. Among intravenous corticosteroid refracter cases, inflixmab and cyclosporine-A became a treatment of severe, acute relapse of UC named “rescue therapy”. Colectomy rate is varying in 38-47% in non-responder patients (10). Probability of colectomy is suggested to be 85% if the number of stools is >8/day or 3-8/day and CRP>45 mg/l on the third day of infliximab or cyclosporine-A rescue therapy (Oxford Index) (10). Sustained fever, persistent bloody diarrhoea and continued CRP elevation on third day of intravenous corticosteroids strongly predicts clinical steroid resistance, thus treatment failure and high risk of colectomy in acute, severe UC (11). On the other hand, patient’s assessment of disease activity could be useful in everyday management. Patient Defined Remission (Higgins et al, 2005) was created by questionnaire using survey question „Is your ulcerative colitis in remission (not active)?” at inclusion. After 1-14 months, at the next visit the patients were asked again whether they are in remission and their UC is better or worse (scale 1–7, where 1 is much better and 7 is much worse). This simple score showed good sensitivity (86%) and specificity (76%) (8).
Walmsley Index or Simple Clinical Colitis Activity Index
Walmsley et al. published a study in which they present a new clinical index for evaluation disease activity in 1998. The Simple Clinical Colitis Activity Index contains clinical signs and symptoms without physician’s global assessment or endoscopic findings; these are the bowel frequency at night and day, urgency of defecation, blood in stool, general wellbeing, extracolonic features (arthritis, pyoderma gangrenosum etc.) (12). Score ranges from 0 to 19 points; although remission was not defined originally, cut-off of <2.5 has been confirmed to correlate with Patient-Defined Remission (13). Walmsley et al. found that this index highly correlates with Powell-Tuck Index and serum albumin, ESR, hemoglobin, hematocrit levels and platelet count (12).
Benefits of SCCAI are that it accurately measures clinical disease activity without requiring direct physicians contact and the addition of the wellbeing question compared to the Mayo Score improves the predictive ability for the patient-reported remission (14). Simple Clinical Colitis Activity Score (or Walmsley Score) shows very good correlation with UC Disease Activity Index (similar to Mayo Score), Lichtinger Index, Seo Index, Endoscopic-Clinical Correlation Index or the Powell-Tuck Index (or St Mark’s Index) which are complex scores incorporating endoscopic features, as well (1).
Mayo Score
The most widely used, but not validated complex clinical and endoscopic index is the Mayo Score described by Schroeder et al. as a part of a clinical trial in 1987 (15). The Mayo Score can be characterized by 4 variables:
a. stool frequency,
b. rectal bleeding,
c. physician’s global assessment and
d. colon mucosa appearance using a flexible endoscope.
Patients’ functional assessment (wellbeing) should be measured involving into physician’s global assessment, as well. Variables are rated 0 to 3 point, thus the range of score is 0 to 12 points. This score is easy to use and more importantly has a good correlation with treatment response and remission. Since these are clinically relevant endpoints, most of clinical trials use the Mayo Score to determine efficacy of therapy and to assess disease outcomes. Mayo Endoscopic Subscore 0 or 1 point was defined as healed mucosa in clinically significant trials like ACT-1, ACT-2, ULTRA-1, ULTRA-2, PURSUIT-SC and PURSUIT-M (16). Criteria for response and remission varies in studies, but generally remission is defined as 0 or 1 point of Mayo Endoscopic Subscore and ≤2 point of total Mayo Score (8). Studies suggest definition of complete response or remission is normal stool frequency, no rectal bleeding, total resolution of well-being, normal endoscopic findings and 0 point of PGA (8). Study reported by Walsh et al. confirm good correlation of Mayo Score with clinical parameters (3). Moreover, Bewtra et al. demonstrated that the 6-point Mayo Score (stool frequency and bleeding) correlated strongly with SCCAI, patient- reported disease activity, partial and full Mayo Score(14, 17).
SEO’s Index
Index described by Seo was developed based on 18 clinical, laboratory and endoscopic variables in 1992 (18). Five variables were defined by multivariable regression analysis, and eventually the Seo’s Index was calculated as 60 × bloody stool + 13 × bowel movements + 0.5 × ESR – 4 × hemoglobin – 15 × albumin + 200. This index significantly predicted response to infliximab or need for colectomy, in addition, correlated with endoscopic findings (8).
The endoscopic scores in ulcerative colitis
The first describer was Bargen in 1937 who assessed the colonic mucosa in colitis (19). He performed observations by rigid proctoscopy with magnifying, and described mucosal changes. In 1955, Truelove and Witts used endoscopic score system to evaluate the mucosal inflammation in clinical trial (20). They used a serial rigid sigmoidoscopy to assess colon mucosa on a 3-point scale:
a. normal or near normal,
b. improved,
c. no change or worse.
This classification neither did not define the mucosal healing or specific changes of inflammation and due to individual judgment of investigators the inter-observer variability was high.
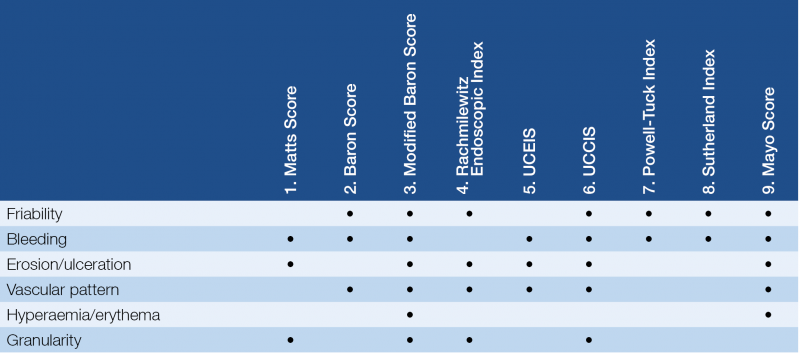
The Matts Score (1961) was based on granularity, bleeding and ulceration (16). The first validated endoscopic index was Baron Index in 1964 (21). In this study 3 investigators performed sigmoidoscopy with assessment of mucosa and it was rated in a 4-point scale. The classification dominantly based on the mucosal vascular pattern, friability and bleeding. Modified Baron Score was developed and used by Feagen et al. in study (9) investigating treatment with humanized antibody to the alpha4beta7 integrin. Scale of 0–4 can be determined by assessing friability, hyperaemia, granularity, vascular pattern, bleeding, ulceration (16). The next significant step on development of endoscopic scores was a 4-point (0–3 degree) scale scoring by Sutherland and Martin in 1987. Use of this index was a part of clinical trial investigating mesalazine enemas. Scoring points increased with the degree of mucosal friability. The Sutherland Index was composed when clinical variables (rectal bleeding, stool frequency, physician’s assessment) were incorporated in this endoscopic score (also known as Disease Activity Index). Study suggests that this index shows a great correlation with patient-defined remission, however, it has been never validated (22). Rachmilewitz et al. define an endoscopic system for evaluation of mucosa in their clinical trial (coated mesalazine versus sulphasalazine) in 1989. The Rachmilewitz Endoscopic Index rates the granularity, vascular pattern, mucosal vulnerability and mucosal damage (0–3 points) and mucosal healing was defined if the sum is ≤4 points (16).
Hanauer et al made endoscopic index evaluating erythema, friability, granularity/ulceration, mucopus and vascular pattern of mucosa (Sigmoidoscopic Index, 1993) (23). Lémann et al and a few years later Hanauer et al. used Sigmoidoscopic Inflammation Grade Score for evaluation endoscopic findings in a study.
None of the currently widely used endoscopic scores consider disease extent therefore it is questionable how much they correlate with the real severity of UC outcome.
In 2015, Lobatón et al. published results about assessment of newly designed endoscopic sore (24). Modified Mayo Endoscopic Score was calculated by assessment of Mayo Endoscopic Subscore in five colonic segments, then the sum was calculated to obtain the Modified Score on a 15-point scale. By multiplying the Modified Score by disease extent in decimeters gives the Extended Modified Score. The MMES was obtained by dividing the Extended Modified Score by the number of segments with active inflammation(24). This score correlated with partial Mayo Score, Geboes Score, CRP and fecal calprotectin (24).
At the same time, our workgroup aimed to develop and assess a new endoscopic score that is a modification of Endoscopic Mayo Subscore. The Pancolonic Modified Mayo Score (25) was calculated with the combination of disease extension and severity. The eMayo Score of the five colorectal segments (ascendending, transverse, descendending, sigmoid colon and rectum) was determined separately and added afterwards. Finally, the sum was multiplied by the Inflammatory Constant if eMayo was ≥2 at least in one segment to clearly distinguish between the active and inactive disease. Score ranges from 0 to 45 points, and has 100% sensitivity and 69.2% specificity. This score significantly correlated with disease extent, partial Mayo Score, Riley Score and serum and fecal inflammatory markers. In addition, Pancolonic Modified Mayo Score showed strong correlation with hospitalization rate, as well (25).
UCEIS: Ulcerative colitis Endoscopic Index of Severity
UCEIS was developed, basically, from Baron Score by Travis et al. in 2012. Due to wide inter-observer variation in endoscopic assessment, a study was performed with 30 investigators rated endoscopic videos for 10 descriptors. Kappa statistics was determined for every descriptor. They found that mucosa friability and bleeding (inter-investigator kappa value 0.40 and 0.37), erosion/ulceration and vascular pattern (inter-investigator kappa value 0.42 and 0.42) had a similar reliability. The final score incorporate 3 descriptors, the range of UCEIS is 0–8 points based on vascular pattern (0–2 points), bleeding (0–3 points) and erosion/ulceration (0–3 points) (2, 26, 27). UCEIS shows good intra-and inter-investigator reliability; in addition, UCEIS accounted for a median 86% of the variability in evaluation of overall severity on the Visual Analog Scale (VAS) when assessing the endoscopic severity of UC among investigators, and UCEIS was unaffected by knowledge of clinical details (26). Despite the weakness of UCEIS, it is an easy to use score that clinicians have began to use and it can be noticed in more and more clinical trials.
UCCIS: Ulcerative Colitis Colonoscopic Index of Severity
Variable components of UCCIS are vascular pattern, granularity, friability, ulceration and global severity of damage. This parameters were evaluated by assessment of 50 colonoscopy video records by 8 expert central reader who scored every segment of colon (22). Results showed good to excellent inter-observer agreement regarding the vascular pattern, granularity, ulceration and global severity of damage (28). Furthermore, there is moderate correlation between UCCIS and serum parameters like CRP (p<0.001), albumin (p<0.001) and hemoglobin (p<0.001), and good correlation between UCCIS and patient reported remission (p<0.001) (22). Samuel et al. validated UCCIS based on full colonoscopy that gives excellent overall assessment of endoscopic severity as judged by the VAS (28).
Summary and conclusions
During the last 60 years several clinical and endoscopic indices have been developed for assessment of UC activity. Indices show heterogenicity not only about descriptors, but also about qualitative features of calculation. The great number of indices could be confusing however, some of them have been validated or have other evidence of clinical utility that may assist in selection. Most of the treatment choices for UC depend on disease activity, and therapy algorithms begin with classifying UC activity as mild, moderate or severe. This is primarily symptom-based at the point of time, which can be covered by scoring systems. Nevertheless, the question arises whether disease activity could be unequivocal, if clinical scores use different descriptors.
In recent years, there is a tendency to use evidence-based score systems, evaluating the colon mucosa by objective parameters. However, despite careful consideration, there are many subjective factors when assessing the condition of the colon mucosa. Feagan et al. suggest that central review of endoscopic images is critical to the conduct of induction studies in UC. One third of patients, who were eligible for enrollment in a study by the site investigator, were excluded after central reading of endoscopic images (29). Walsh et al. found that agreement between specialists was 89% for SCCAI, 83% for Mayo Score and 95% for Seo Index. This means that 1 in 5 patients might be excluded from an endpoint in clinical trials due to inter-observer variation. In a case of SCCAI it was 1 in 9 of Seo was 1 in 20 patients, respectively. However, inter-observer variation was good for these indices if we compared them to validated Modified Baron Index as reference. Kappa values were for the SCCAI, Mayo Score and Seo Index 0.75, 0.72 and 0.89 contrary to Modified Baron Index with 0.44 of kappa statistics (30).
In conclusion, „perfect” or optimal clinical scoring system is not available for UC, but some of them have a great evidence of clinical utility. Mucosal healing became therapeutic endpoint due to its correlation with short-and long-term favourable disease outcome, therefore reliable, responsive, predictive, feasible and validated tools are needed to define mucosal status.
No conflict of interest.
1. Peyrin-Biroulet L, Panes J, Sandborn WJ, Vermeire S, Danese S, Feagan BG, et al. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2016; 14(3): 348–354.e17.
2. Walsh A, Palmer R, Travis S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin N Am 2014; 24(3): 367–78.
3. Walsh AJ, Ghosh A, Brain AO, Buchel O, Burger D, Thomas S, et al. Comparing disease activity indices in ulcerative colitis. J Crohns Colitis [Internet] 2014; 8(4): 318–25. Available from: http://dx.doi.org/10.1016/j.crohns.2013.09.010
4. Truelove S, Witts L. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955; 2: 1041–8.
5. Dinesen LC, Walsh AJ, Protic MN, Heap G, Cummings F, Warren BF, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis 2010; 4(4): 431–7.
6. Powell-Tuck J, Bown R, Lennard-Jones JE J. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol 1978; (13): 833–7.
7. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317(26): 1625–9.
8. D’Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, et al. A Review of Activity Indices and Efficacy End Points for Clinical Trials of Medical Therapy in Adults With Ulcerative Colitis. Gastroenterology 2007; 132(2): 763–86.
9. Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JWD, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med 2005; 352(24): 2499–507.
10. Jarnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005; 128(7): 1805–11.
11. Lindgren SC, Flood LM, Kilander AF, Lofberg R, Persson TB, Sjodahl RI. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol 1998; 10(10): 831–5.
12. Walmsley R, Ayres R, Pounder R, Allan R. A simple clinical colitis activity index. Gut 1998; 43(1): 29–32.
13. Higgins P, Schwartz M, Mapili J, Krokos I, Leung J, Zimmerman E. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005; 54: 782–8.
14. Bewtra M, Brensinger CM, Tomov VT, Hoang TB, Sokach CE, Siegel CA, et al. An Optimized Patient-reported Ulcerative Colitis Disease Activity Measure Derived from the Mayo Score and the Simple Clinical Colitis Activity Index. Inflamm Bowel Dis. 2014; 20(6): 1070–8.
15. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317(26): 1625–9.
16. Paine ER. Colonoscopic evaluation in ulcerative colitis. Gastroenterol Rep 2014; 2(3): 161–8.
17. Bennebroek Evertsz’ F, Nieuwkerk PT, Stokkers PCF, Ponsioen CY, Bockting CLH, Sanderman R, et al. The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the. J Crohns Colitis 2013; 7(11): 890–900.
18. Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol 1992; 87: 971–6.
19. Bargen J. The medical management of chronic ulcerative colitis: (section of surgery: sub-section of proctology). Proc R Soc Med 1937; 30: 351–62.
20. Truelove S, Witts L. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955; 2: 1041–8.
21. Baron J, Conell A, Lennard-Jones J. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J 1964; 1: 89–92.
22. Samaan MA, Mosli MH, Sandborn WJ, Feagan BG, D’Haens GR, Dubcenco E, et al. A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm Bowel Dis 2014; 20(8): 1465–71.
23. Hanauer S, Schwartz J, Robinson M, Roufail W, Arora S, Cello J, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol 1993; 88(8): 1188–97.
24. Lobaton T, Bessissow T, De Hertogh G, Lemmens B, Maedler C, Van Assche G, et al. The Modified Mayo Endoscopic Score (MMES): A New Index for the Assessment of Extension and Severity of Endoscopic Activity in Ulcerative Colitis Patients. J Crohns Colitis. 2015; 9(10): 846–52.
25. Balint A, Farkas K, Szepes Z, Nagy F, Szucs M, Tiszlavicz L, et al. How disease extent can be included in the endoscopic activity index of ulcerative colitis: the panMayo score, a promising scoring system. BMC Gastroenterol 2018; 18(1): 7.
26. Travis SPL, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel J, et al. Reliability and Initial Validation of the Ulcerative Colitis Endoscopic Index of Severity. Gastroenterology 2013; 145(5): 987–95.
27. Samuel S, Bruining DH, Loftus EVJ, Thia KT, Schroeder KW, Tremaine WJ, et al. Validation of the ulcerative colitis colonoscopic index of severity and its correlation with disease activity measures. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2013; 11(1): 49–54.e1.
28. Feagan BG, Sandborn WJ, D’Haens G, Pola S, McDonald JWD, Rutgeerts P, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology 2013; 145(1): 149–157.e2.
29. Walsh AJ, Ghosh A, Brain AO, Buchel O, Burger D, Thomas S, et al. Comparing disease activity indices in ulcerative colitis. J Crohns Colitis 2014 prilis; 8(4): 318–25.
