How do you like the pen?
Summary
Inflammatory bowel diseases have a huge impact on the quality of life and workability. The fully human anti-TNF-alpha, adalimumab provides great opportunity to use biological therapy at home, application of the prefilled syringe might be difficult for some. The aim of this study was to evaluate patients’ opinion about the newly introduced adalimumab prefilled pen compared to the prefilled syringe. Our questionnaire based survey focused on the degree of difficulty of the usage, any inconveniences and the proportion of self-injection for each mode of administration.
Introduction
Inflammatory bowel diseases (IBD) are chronic, relapsing, multiorgan conditions with unknown aetiology and decreased quality of life (QoL). Growing knowledge about the aetiology led to therapeutic breakthrough during the last decades in IBD. Targeted biological agents against TNF-alpha (tumor necrosis factor-alpha) proved to be the most effective treatment option even in the most challenging and complication associated cases. Infliximab, adalimumab and golimumab are the approved anti-TNFs for the treatment of different forms of IBD. Administration of these agents is markedly varied and adalimumab is the only one which is administered via subcutaneous (SC) injection using either a prefilled syringe or an autoinjection pen. Both devices are single-use, disposable, prefilled and adequate for self-administration. The autoinjection pen contains 40 mg adalimumab in 0.8 mL solution, like the prefilled syringe and was approved by the US Food and Drug Administration (FDA) based on a safety and bioavailability study comparing the syringe and the autoinjection pen in 295 healthy volunteers (1).
Self-administered injectable medications have some advantages of portability, flexible scheduling, and lower costs over intravenous infusions, however the risk of non-adherence is higher and problems relating to injection technique may occur in certain patients (2), furthermore, not all patients are physically able to inject themselves. Non-adherence is considered as a major problem among patients with chronic, incurable disease, and the World Health Organization claimed in 2003 that improving patient adherence to long-term therapies would be more beneficial than any other intervention (3).
Autoinjection pens have been shown to be preferred over syringes by patients requiring long term SC administration of medications in other chronic conditions, like diabetes mellitus, migraine, and growth hormone deficiency (4).
Concerning the administration of adalimumab by two different ways, by two single-use injection devices, up to now two studies were performed in rheumatoid arthritis (RA) with relatively low number of patients. Both of them confirmed that patients preferred the pen versus the traditional prefilled syringe because they found it easier to use and to felt less pain during the administration (5, 6).
However, patients with RA differ from patients with IBD in the age and the RA associated functional limitations which are uncommon among younger IBD patients and results in difficulties in subcutaneous self-administering. Therefore the objectives of our multicentre study were to evaluate patients’ opinion about the newly introduced adalimumab prefilled pen compared to the prefilled syringe.
Patients and methods
This was a prospective, multicentre, questionnaire-based observational study carried out in eleven Hungarian IBD centres. All patients diagnosed with ulcerative colitis (UC) or Crohn’s disease (CD) enrolled in the study were treated with adalimumab and changed syringe to autoinjection pen so they had enough experience with both methods. The study design was a simple observational study the switch was performed independently after Abbvie started to market autoinjection pen formulation instead of syringe at November 2016 in Hungary.
Adult patients diagnosed with UC or CD, who have had experiences with both forms of adalimumab, performed the questionnaire-based survey anonymously. Inclusion criteria were also age over 18 years, IBD diagnoses based on symptoms, radiological, endoscopical, and histological findings at least three months prior to the study. All patients were selected voluntarily. Patients who did not meet the above-mentioned inclusion criteria were excluded. All of the patients were on a 40 mg subcutaneous maintenance adalimumab therapy in every other week and had experiences with both form of adalimumab subcutaneous injection; the prefilled syringe and the prefilled autoinjection pen.
Online and paper-based surveys were performed. The survey was based on the differences between the prefilled syringe and the prefilled autoinjection pen; questions were about the difficulty of administration, inconveniences with administration with each form of adalimumab. Specifically, the patients had to rate the difficulty of administration in a visual analog scale (VAS, easy to use = 1 to very difficult to use=10) and to write comments on their own words. The questionnaire contained questions for patients’ demographic data (gender, age, patients’ highest educational level and occupational status), IBD characteristics (year of diagnosis, phenotype, concomitant medication – previous biological therapy. Further questions of the questionnaire are in Appendix 1.

Statistics
Statistical analysis was performed using R Commander statistic programme, Khi2-test, Sign-test, McNemar-test, Marginal homogenity-test, Mann-Whitney-test and Fisher-test in collaboration with statisticians. Baseline characteristics and other categorical data were summarized using means. An “exact” 95% coinfidence interval (CI) was performed to compare patients who preferred the pen with patients who preferred the prefilled syringe. All statistical tests were performed at the 0.05 tx level of statistical significance.
Ethical approval
This study was approved by the Clinical Research Coordination Center University of Szeged Faculty of Medicine Albert Szent-Györgyi Health Center.
Number of ethical licence: 4005.
Results
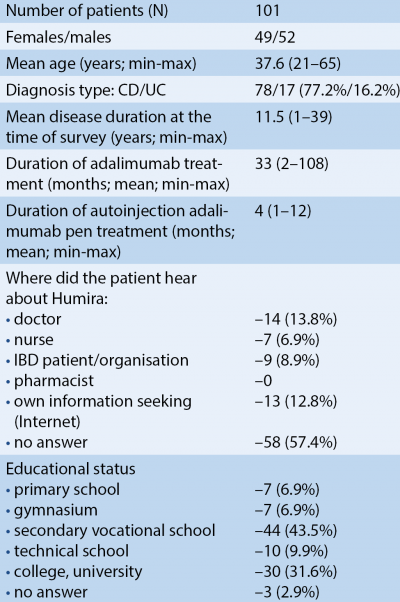
A total of 101 patients were enrolled in our multicenter study. The baseline characteristics of the patients are reported in Table 1. Fifty-two men and 49 women were enrolled in the study; mean age at the time of the survey was 37.6±11.3 years. Seventy-seven point two % of the patients were diagnosed with CD and 16.2% with UC. Mean duration of adalimumab treatment was 33 months (2-108 months) and the mean duration of autoinjection pen treatment was 4 months (1-12 months).
Sixty-seven of 97 (69.1%) patients chose adalimumab therapy because their doctor advised it, 27 (27.8%) patients because the previous biological therapy was ineffective or they had an allergic reaction previously and 3 (3.1%) because they found it to be easier to use and required less time to inject.
The majority of the patients (97/100; 97%) were satisfied with the effectiveness of the adalimumab treatment in general and only 3% felt it ineffective. Forty-five of 78 (58%) CD patients and 4 of 17 (23%) UC patients felt that their therapy is effective and all of the symptoms vanished (p=0.017). According to our results the number of those patients who were satisfied with their therapy was significantly higher among CD patients. Examining the efficacy of adalimumab therapy we found that the previous other type of biological therapy (p=0.04) and the type of the diagnosis (CD or UC, p=0.017) affected significantly the feeling of effectiveness of the recent therapy (p=0.04). After switching from the prefilled adalimumab syringe to the autoinjection pen the percentage of patients who gave the injection themselves increased from 66% (66 of 100 patients) to 95% (95 of 100 patients) (p<0.001).
Moreover, 19% of the patients found the prefilled syringe caused difficulties and 15% had serious aversion to administer it to themselves. In contrast with the subcutaneous injection form, 94% of the patients thought that the autoinjection pen is practical and easy to use and only 1% had serious aversion to use it. In this study, the pen showed a statistically significant advantage regarding the mode of the administration, in the injection pain, and the difficulty of administration (p=0.05).
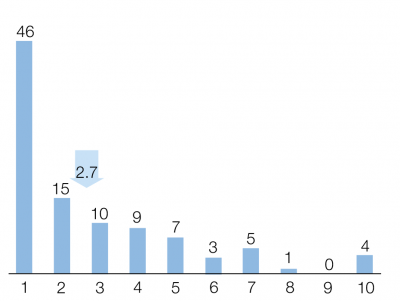
Regarding preference, patients found significantly easier to inject pen over syringe. The mean (sd) visual analogue scale (VAS) score was 2.78 (2.37) for the syringe compared with 1.57 (1.26) for the pen (p < 0.001). Figure 1 and 2 shows the detailed data. In this study we found that the level of the difficulty of the administration the syringe or the pen was independent from the level of education. (p=0.268 and p=0.587). No association was shown between gender or type of diagnosis and the difficulty of the drug administration. Overall 70% of the patients assessed the switch positively, and they reported significantly less inconvenience with administration (23.6% vs. 9.3%; p=0.09) (Figure 3).


Discussion
This was the first study examining the ease of use of prefilled adalimumab syringe and autoinjection pen in a large series of IBD patients. The previous data, coming from RA studies concerning that the administration of the pen is less painful over syringe, when the disease itself can cause functional limitations to subcutaneous self-administering and that is why the mode of administration can be more highlighted. Our study proved that autoinjection pen is more comfortable and more favour in the group of younger IBD patients without disability. The pen decreased the aversion from autoinjection from 15 to 1%, and while only 66% of the patients administered the syringe themselves before, 95% did it after switching to the pen. Educational level, gender and the type of the disease was not in association with the patients’ opinion. After switching to the pen, patients reported significantly less pain and less local skin reactions, and felt significantly easier to inject.
The TOUCH study was the first examination in this topic involving 52 patients with RA in 2006. Although both studies (TOUCH and our) used different, self-made questionnaires, some of the results are highly comparable. Eighty-eight point five % of the RA patients preferred the pen in the TOUCH study; while 94% of the IBD patients thought that the autoinjection pen is practical and easy to use while only 1% of the IBD patients had serious aversion with it (5). Overall, 70% of our patients assessed the switch to pen positively and only 9.3% of the patients felt any inconveniences regarding the pen.
Self-administering of the medication is one of the most important factors which increases patients’ adherence and reduces excessive workloads in primary care. The study Borras-Blasco J et al. about the acceptability of switching adalimumab from a prefilled syringe to an autoinjection pen examined this very important factor in 55 patients with different rheumatoid diseases. The percentage of patients self-administering medication increased from 51 to 84% in this study, while 66 to 95% in our study. The explanation of the higher level observed in our study can be the younger age (37.4 vs. 50 years) of the IBD patients. Although this comparison may suggest that younger patients are braver to inject themselves, the introduction of a simpler and less uncomfortable autoinjection technique may increase the self-administering rate independently of the age of the patients. The mean visual analogue scale score was significantly lower after switching in both studies: 3.52 (2.26) for the prefilled syringe compared with 2.02 (2.16) for the autoinjection pen among patients with rheumatoid disorders and 2.78 (2.37) for the syringe compared with 1.57 (1.26) for the pen among IBD patients (6).
To summarize, our multicenter study including more than hundred IBD patients revealed that after switching to pen from the prefilled syringe results a higher self-administering rate and significant reduction in pain and local reactions at the injection site. Our study provides new evidence to support the use of the adalimumab autoinjection pen in another group of patients with chronic disorders as previously observed in IBD.
The authors report no conflicts of interest.
Funding source: This work was supported by the research grants of the National Research, Development and Innovation Office (Grant ID: 119809 and 125377), by the UNKP-17-4 New National Excellence Program of the Ministry of Human Capacities and by the EFOP-3.6.2-16-2017-00006
András Szijártó was supported by the European Union and the State of Hungary, co-financed by the European Social Fund within the framework of the EFOP-3.6.1-16-2016-00008.
2. Schwarzenbach F, Dao Trong M, Grange L, Laurent PE, Abry H, Cotten J, Granger C. Results of a human factors experiment of the usability and patient acceptance of a new autoinjector in patients with rheumatoid arthritis. Patient Preference and Adherence 2014; 8: 199–209.
3. World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization, 2003.
4. Rapaport R, Saenger P, Schmidt H, Hasegawa Y, Colle M, Loche S, Marcantonio S, Bonfig W, Zabransky W, Lifshitz F. Validation and ease of use of a new pen device for self-administration of recombinant human growth hormone: results from a two-center usability study. Medical Devices: Evidence and Research 2013; 6: 141–146.
5. Kivitz A, Cohen S, Dowd JE, Edwards W, Thakker S, Wellborne FR, Renz CL, Segurado OG. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther 2006; 28: 1619–29.
6. Borras-Blasco J, Garcia-Perez A, Rosique-Robles LD, Castera DE, Abad J. Acceptability of switching adalimumab from a prefilled syringe to an autoinjection pen. Expert Opinion on Biological Therapy 2010; 10: 301–307.
