Are the left- and the right-sided colon cancers similar?
Summary
Colorectal carcinoma (CRC) is a heterogeneous disease (CRCs) displaying variable etiology, incidence, pathobiology and related molecular pathways, and most importantly, the outcome varies depending on the location of the tumor. Several studies have been conducted which shed light on differences in overall survival between right-sided (RCRC) and left-sided CRC (LCRC). Likely the differences between RCRC and LCRC are due to complex mechanisms of genetic and epigenetic changes caused by intrinsic and extrinsic factors. Therefore, it is essential to determine the individual variations in biological and molecular characteristics of CRCs and consecutively treat cancer patients in a personalized fashion.
A colorectalis carcinoma egy heterogén betegség (CRC), amely változó etiológiát, incidenciát, patobiológiát mutat, és ezekhez kapcsolódó molekuláris útvonalakat érint. Ami a legfontosabb, a kimenetel a daganat helyétől függően is változik. Számos olyan vizsgálatot folytattak le, amelyek kimutatták a jobb oldali és a bal oldali CRC közötti túlélésbeli különbségeket. Valószínűleg a jobb és bal oldali CRC közötti különbségek intrinzik és extrinzik tényezők által okozott genetikai és epigenetikai változások komplex mechanizmusa miatt következnek be. Ezért alapvető fontosságú a CRC-k biológiai és molekuláris változatainak meghatározása és a tumoros betegek személyre szabott, ezt is figyelembe vevő kezelése.
Epidemiology and a brief pathology
Worldwide, approximately 8.5% of all cancers are malignant epithelial tumours of the colon. A wide variation in the incidence can be detected, because in the high-risk developed countries (Europe, the Americas, Australia, New Zealand) the incidence is 20-times higher than in the low risk regions (Africa, Asia) (1). In Europe, colorectal cancer is the second most commonly diagnosed cancer and the leading cause of death (2). Despite that the clinical outcome of colorectal cancer patients has highly improved by innovative treatment options and development of liver surgery with ablative technics, the prognosis of metastatic colorectal carcinoma (mCRC) remains poor (2).
In addition, there is a massive difference in the age of
onset of CRCs, the mean age being only 50 years in developing countries. The lifestyle risk of developing CRCs is nearly 6% in US and 7.6% in Europe (1).
The risk factors of colorectal cancers can be found in Table 1. Although the prognosis is stage and grade dependent, tumours with identical morphological features display considerable heterogeneity in clinical outcome.
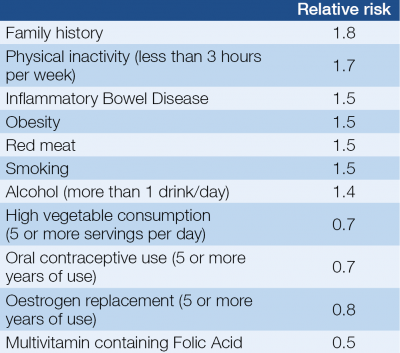
In central Europe, and of course in Hungary, the development of colorectal cancer is predominantly a disease of late middle-aged and elderly individuals.
It is necessary to highlight that there is inverse correlation in vegetable and fibre consumption and the incidence of CRCs. It is well known that high folate intake can decrease the risk of colorectal cancer and the effect of Vitamin D likely same.
On the other hand, alcohol intake has been associated with an increased risk of colorectal cancer. Despite that the tobacco-related cancers’ number is getting higher the relative risk of colorectal cancer in smoking population is much less. Due to the lifestyles, there has been a trend in recent years toward the occurrence of more proximal cancers (1, 3).
Embryology is the background of the difference between the left and right colon. The intestinal tract from the proximal duodenum to the two-third of the transverse colon originates from the midgut. Rest of the colon transversum until the proximal part of anal canal develops from the hindgut. If we see the metabolic activity of the entire colon, active metabolism can be found in the right side compared to the silent metabolic activity of the left colon. It seems to be that there is a biochemical and the functional alteration between the left and right colon (4).
To sum up the behaviours, RCRCs show more advanced TNM stages with larger tumour sizes and dominantly mucinous features. They manifest with a female predominance in the population with increasing incidence. Microsatellite instability and BRAF mutations are often seen. Unfortunately, the overall survival is shorter compared with LCRCs. Compared with the right side, LCRC incidences are decreasing slowly, but two-thirds of the CRCs are located at this side. With a male predominance in the population, the TNM staging is more favourable with smaller tumour sizes (5).
Nowadays, the hot topic is the biomolecular differences between the right and left sided CRC, and for this reason, next we discuss the biological behaviours of the left and right sided CRCs.
Brief summary of clinical features
The change in bowel habit, constipation, abdominal distention, haematochezia, or tenesmus are the well-known symptoms of the CRC, especially for the rectosigmoid lesions. Some patients have only general symptoms, such as weight loss, fever, malaise or anaemia. Only roughly 40% of the patients have localised disease, the other 40% have regional metastasis, and one fifth of the CRC patients present with metastases at the time of primary diagnosis (3, 6).
Several studies have found high concordance rate for mutations in the driver oncogenes RAS and BRAF.
For the clinical oncologists, the presence of activated mutation of the KRAS (are presented in about 40% of CRC), and NRAS (similar to KRAS in structure and implications, and is activated by somatic mutation in about 7% of colorectal carcinomas) are most important. Nowadays, BRAF mutation has become important in daily routine.
Microsatellite instability is characterized by widespread alterations in the size of repetitive DNA sequences, caused by defective DNA mismatch repair. There are high (MSI-H) or low levels of instability (MSI-L); tumours with MSI-H tend to be more proximally located, poorly differentiated. MSI can be seen in 10-15% of sporadic carcinomas and hereditary nonpolyposis colon cancers (7, 8).
Paradigm of precision medicine
Improved stratification of colorectal carcinoma based on RNA expression studies, encompassing tumor, stroma and infiltrating immune components, has revealed convergent pathway abuse in CRC that imply a “multi-molecular” perspective for the development of therapies to treat tumors of the large intestine. That was the basis of the consensus molecular subtypes of CRC in 2017. Gene expression properties result in phenotypic and outcome measures, which have been extensively used to identify biologically homogeneous groups of tumors. This resulted in a consensus molecular classification that allows classification of cancers into one of four robust subgroups (9). The four consensus molecular subtype (CMS) groups represent the current best description of CRC heterogeneity at the gene-expression level (9, 10). For better understanding see Figure 1.
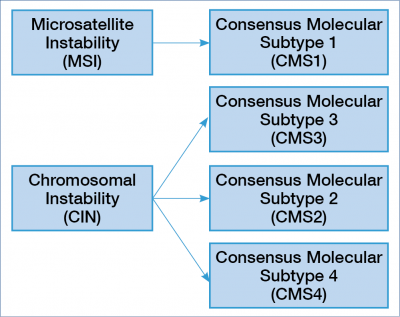
There is an association between the genomic, epigenomic, transcriptomic pathways and the stromal immune microenvironment, the driver genes and the clinical outcome. It seems that the highly immunogenic tumors are associated with immune activation and the MSI status. In the clinical practice, we have the opportunity to insert immuno-oncology treatment into the treatment algorithm. In this group, the role of the driver genes is emphasised, and these tumours are predominantly proximally localised (9, 11).
All studies so far confirmed the extensive inter-tumour heterogeneity of CRC at the genomic, epigenomic, transcriptomic and immune levels.
The MSI group (CMS1) is more homogenic compared to the chromosomal instability (CIN) group, which is highly heterogeneous and divided into three consensus molecular subtypes. Tumours with CIN are mainly localized in the left colon / rectum, and the microenvironment is either poorly immunogenic or inflamed, with marked stromal infiltration. The “epithelial canonical” group named CMS2 the “mesenchymal” group named CMS4. The group CMS3 has a strong metabolic adaptation and is enriched for RAS mutation (9). Based on these new categories, we will know more and more about the primary resistance and the acquired resistance, which develops during treatment (9, 12).
In the everyday practice, HER2 receptor was the first biomarker for targeted therapy; later, retrospective analysis of metastatic CRC trials presented an association at first with KRAS exon 2 mutation and innate resistance to anti-EGFR therapy. Then, the determination of KRAS was combined with NRAS, and now we use RAS wild type and RAS mutated stratification in CRC. It is important, because treatment guidelines have inserted this stratification using as a key set of biomarkers for standard of care management (9, 12).
The EGFR monoclonal antibodies producing clinical benefit for those patients who has wild type RAS status when added to standard chemotherapy (9, 13).
Clinical trials – meta-analysis
The result of a meta-analysis can transmit a lot of information in a short, digestible way for a physician. A German working group analysed 13 first-line randomized trials and 1 prospective pharmacogenic study, and found that the right and the left sided colorectal cancers were different (14).
The meta-analysis of two large studies (PRIME and CRYSTAL: the control arm was only chemotherapy without biologicals) highlighted that primary tumour location was predictive for survival benefit, from addition of anti-EGFR antibody to standard chemotherapy if the patient had RAS wild tumour type (15). The analysis of other studies showed that RAS wild type left-sided cancer had a significantly better survival benefit from anti-EGFR medication, compared to anti-VEGFR treatment plus standard chemotherapy (16). It has been suggested that the primary tumour location has prognostic influence and impact on response to biological therapy in metastatic CRC (17). Of course, prospective studies are needed with large patient numbers, but from this retrospective analysis, the conclusion is that left sided CRC appears to have a positive predictive factor for survival benefit from anti-EGFR treatment in patients with RAS wild type cancer. (It means that cetuximab or panitumumab plus standard chemotherapy is the standard option). In the right-sided colon disease, the anti-VGEFR-based therapy showed more favourable outcomes. There is a suggestion for using anti-EGFR plus chemotherapy combination for the right side tumour if it is “triple wild” (KRAS, NRAS, BRAF wild-type), but this suggestion requires further investigation.
Are the right and the left colon cancers different?
Patient with right side disease and left side cancer differ in their clinical characteristic, clinical outcome and response to treatment because of different molecular profiling and microbiome. Nowadays, we cannot determine the reason or the driver of these differences. Despite that we have a guideline for using of anti EGFR antibodies in subgroup of metastatic CRC with RAS wild type, in the left side disease the standard remains as the EGFR plus chemo combina-
tion, while in the right side disease the RAS wild type group may benefit more from upfront anti-VEGFR and chemotherapy combination (except in case the tumour is BRAF wild too) (2, 13, 18).
It was showed that the relative benefit from FOLFOXIRI and bevacizumab combination over FOLFIRI plus bevacizumab is much more pronounced in right-sided tumours independently their RAS, BRAF status (19).
About 10 years ago, based on the analysis of the SEER database’s information available, it was found that right-sided colon cancers have a worse prognosis than left-sided colon cancers. The reason for this is still unclear, but it might be due to biological and environmental factors, and may have particular bearing, given the rising incidence of right-sided colon carcinomas.
New paradigm, which considers left and right colon cancer as distinct clinical entities when performing international comparisons on cancer treatment and survival, concludes that CRC should be stratified according to tumor location, whether proximal or distal to the splenic flexure (20).
2. Stintzing S, Tejpar S, Gibbs P, et al. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer 2017; 84: 69–80.
3. Hornick JL, Odze RD. CHAPTER 19 – Polyps of the Large Intestine. In: Odze RD, Goldblum JR, editors. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas (Second Edition). Philadelphia: W.B. Saunders; 2009. p. 481–533.
4. Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). Journal of Clinical Oncology 2016; 34(suppl 15): 3504.
5. Lee GH, Malietzis G, Askari A, et al. Is right-sided colon cancer different to left-sided colorectal cancer? – a systematic review. Eur J Surg Oncol 2015; 41(3): 300–8.
6. Redston M. CHAPTER 23 – Epithelial Neoplasms of the Large Intestine. In: Odze RD, Goldblum JR, editors. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas (Second Edition). Philadelphia: W.B. Saunders; 2009. p. 597–637.
7. Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. Journal of clinical pathology 2008; 61(5): 561–9.
8. Wright CL, Stewart ID. Histopathology and mismatch repair status of 458 consecutive colorectal carcinomas. Am J Surg Pathol 2003; 27(11): 1393–406.
9. Dienstmann R, Vermeulen L, Guinney J, et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 2017; 17(4): 268.
10. Dienstmann R, Rodon J, Barretina J, Tabernero J. Genomic medicine frontier in human solid tumors: prospects and challenges. J Clin Oncol 2013; 31(15): 1874–84.
11. Dienstmann R, Jang IS, Bot B, et al. Database of genomic biomarkers for cancer drugs and clinical targetability in solid tumors. Cancer Discov 2015; 5(2): 118–23.
12. Dienstmann R, Tabernero J. Cancer: A precision approach to tumour treatment. Nature 2017; 548(7665): 40–1.
13. Dienstmann R. Tumor Side as Model of Integrative Molecular Classification of Colorectal Cancer. Clin Cancer Res 2018; 24(5): 989–90.
14. Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer 2017; 70: 87–98.
15. Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017; 28(8): 1713–29.
16. Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol 2017; 3(2):194–201.
17. Giuliani J, Bonetti A. Epidermal growth factor inhibitors in first-line for metastatic colorectal cancer with ras wild-type: a perspective based on pharmacological costs. Expert review of pharmacoeconomics & outcomes research 2017; 17(3): 243–8.
18. Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018; 29(1): 44–70.
19. Cremolini C, Antoniotti C, Lonardi S, et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer. Retrospective analysis of the TRIBE trial by GONO. Ann Oncol 2018; 29(7):1528–34.
20. Meguid RA, Slidell MB, Wolfgang CL, et al. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol 2008; 15(9): 2388–94.
