Kongresszusi összefoglaló / Congress report
Gastro Update Europe 2020 Meeting report of Gastro Update Europe 2020, September 4–5, 2020
Summary
This narrative review summarizes a selection of clinically-important novel gastrointestinal developments, presented and discussed at the virtual Gastro Update Europe.The selected topics, fully referenced, reflect what the distinguished faculty considered of vital importance to be communicated to the astute busy gastro-hep clinician who is eager to stay abreast of important novel developments. Whenever appropriate a personal comment or addition was added to further raise the educational value of this review. Given its narrative character, statements and conclusions are largely expert opinion-based, and referencing within this summary is limited to the included slide images.
Summary
This narrative review summarizes a selection of clinically-important novel gastrointestinal developments, presented and discussed at the virtual Gastro Update Europe.The selected topics, fully referenced, reflect what the distinguished faculty considered of vital importance to be communicated to the astute busy gastro-hep clinician who is eager to stay abreast of important novel developments. Whenever appropriate a personal comment or addition was added to further raise the educational value of this review. Given its narrative character, statements and conclusions are largely expert opinion-based, and referencing within this summary is limited to the included slide images.
Novel upper gastrointestinal developments –Peter Malfertheiner (Germany)
The prevalence of gastroesophageal reflux disease (GERD) is rising worldwide, peaking in the elderly. Newly added to the traditional modalities to diagnose GERD (endoscopy/biopsy; high resolution manometry; pH monitoring; pH-Impedance monitoring) is mucosal impedance testing, either by probe or balloon-based technology. The balloon mucosal impedance catheter detects changes in esophageal mucosal integrity over a 10 cm long segment of the esophagus during endoscopy. The high impedance of the normal healthy mucosa falls as mucosal inflammation and barrier disturbance develops. Such technology may reduce the need for prolonged ambulatory wireless pH monitoring.
A newcomer in pharmacotherapy is a non-bicarbonate containing alginate, which was shown in monotherapy to relieve reflux symptoms in patients without esophagitis, comparable to proton pump inhibitors (PPIs). The efficacy of the novel acid blocker (p-CAB) vonoprazan in alleviating symptoms and healing of esophagitis was confirmed in several Asian countries and shown to be at least equipotent to PPI. So far, no data from Europe have been presented. If and when p-CABs will be available in Europe is uncertain.
Mangement of heartburn during pregnancy and lactation always causes some concern. Mild symptoms may be adequately treated with antacids, alginates or sucralfate. If symptoms persist, H2RAs can be used. PPIs (classified as ‘low risk’) have been used, even during the first trimester, but a recent systematic review revealed that PPI use was associated with an increased risk of congenital malformations with an Odds Ratio (OR) of ~2 in case-control studies.
PPI-non responsive or PPI refractory GERD occurs in at least ~30% of patients. This was confirmed in a recent American population-based study, showing that 54% of 3.229 participants taking daily PPIs had persistent GERD symptoms. When analysing such data, it is obviously essential to discriminate functional heartburn from GERD. An expert review panel defined functional heartburn as retrosternal burning pain or discomfort which persists despite maximal PPI therapy, taken appropriately before meals during a 3-month period. Instead of PPI such patients are better treated with tricyclic antidepressants or selective serotonin reuptake inhibitors. Whether patients with PPI-refractory heartburn can profit from intra-lower-esophageal sphincter application of radiofrequency energy (Stretta) was evaluated in a sham-controlled trial but the outcome was negative. Perhaps more interesting are the results obtained by adding a novel bile acid sequestrant as adjunct to PPI in refractory patients. In a placebo-controlled trial involving 280 patients, the weekly heartburn severity score decreased almost 60% by adding 1500 mg of the bile acid binder which was well tolerated – also, regurgitation improved. More studies are awaited to confirm these intriguing findings. Also, the more potent acid blocker vonoprazan was evaluated in 124 patients with ongoing symptoms and abnormal esophageal acid exposure despite appropriate PPI therapy. Vonoprazan led to normalisation of esophageal acid exposure and significantly improved symptoms and healed erosive esophagitis.
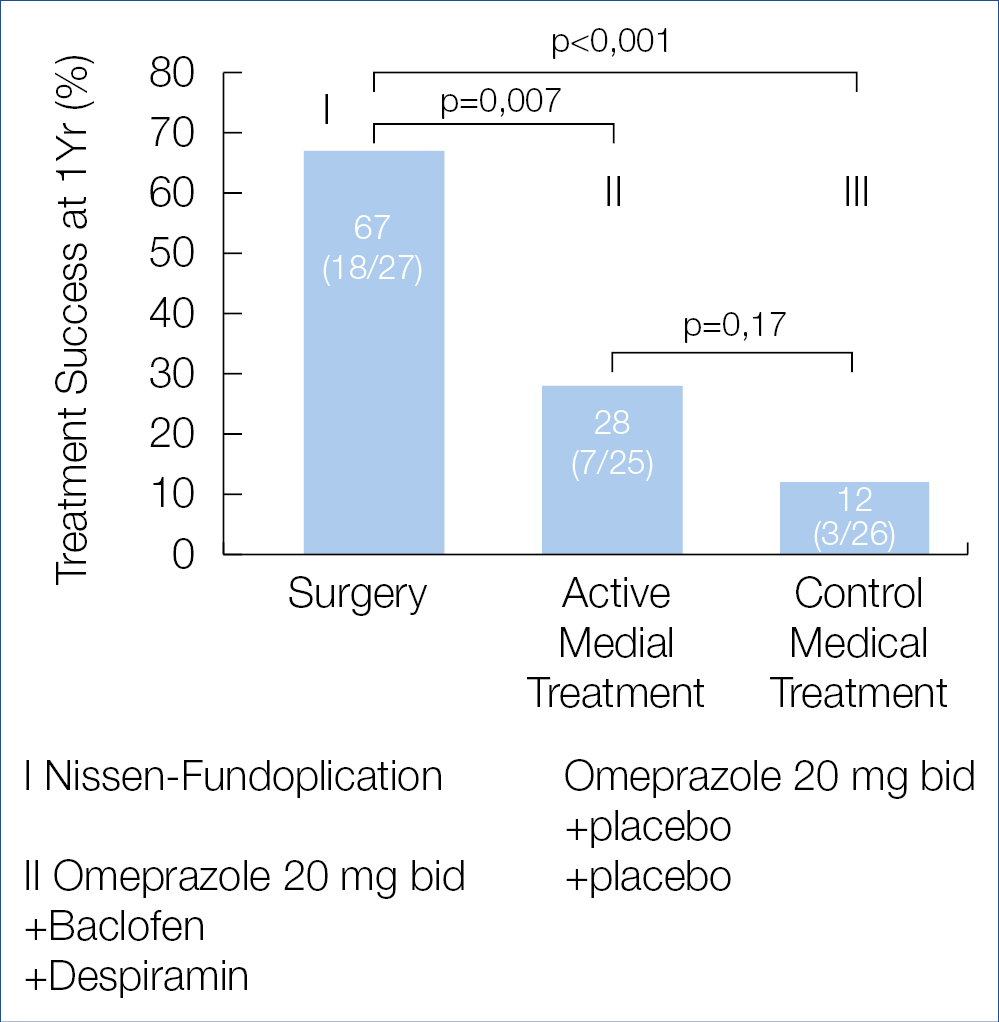
Is there a place for surgery for patients with refractory heartburn? This was evaluated in a randomized American trial involving 78 patients, comparing laparoscopic Nissen fundoplication to omeprazole + baclofen + despiramin or to omeprazole monotherapy. The results published in the New England Journal of Medicine are shown below, indicating that fundoplication may benefit a small highly-selected subset of refractory patients. Whether the novel non-surgical modalities such as transoral incisionless fundoplication or electrical sphincter stimulation can mimic the shown surgical results is not known. (Figure 1.) Adequate diagnosis of GERD and its distinction from func-tional or hypersensitivity-related heartburn and adequate therapeutic recommendations becomes increasingly challenging for the practicing clinician, confronted with a plethora of diagnostic and therapeutic possibilities and looking for clinical guideline help.
Esophageal columnar metaplasia or Barrett Esophagus (BE) is a well known risk factor for esophageal adenocarcinoma but the vast majority of cancer patients have no prior diagnosis of BE. To raise the detection rate of BE, a randomised trial was conducted in British general practice clinics involving 13.514 patients on long-term acid suppressant therapy comparing usual care versus investigation with esophageal cytology using the cytosponge-trefoil factor procedure, followed by endoscopy if trefoil factor positive cells were found. One year after enrolment, BE was diagnosed in <1% in the control group versus 2% in the intervention group which included a few patients with dysplasia and cancer. Other modalities for detection of BE are also being explored and time will tell which one is ultimately clinically useful. Some investigators even voice the possibility of combining screening colonoscopy with upper intestinal endoscopy looking for esophageal and gastric mucosal metaplasia.
Eosinophilic Esophagitis (EoE) remains a rich source of basic and clinical research. The traditional therapeutic possibilities include dietary allergen exclusion, PPIs and various topical corticosteroids such as budesonide and fluticasone. The efficacy of 2 × 1 mg oral viscous budesonide was compared to 2 × 880 mcg fluticasone multidose inhaler in a controlled 8 week trial. Both products showed a comparable improvement in mucosal eosinophil count, endoscopy and dysphagia score. Note that the list of topical cortocosteroids now includes budesonide orodispersible tablets and fluticasone orally disintegrating tablets. In a network meta-analysis of 17 controlled trials, involving 1.011 patients and 15 therapeutic modalities, 2 × 1 mg budesonide orodispersible tablets turned out to be most effective therapy in adults whereas fluticasone was the preferred drug in children.
Another therapeutic novelty is a monoclonal antibody (RPC4048) against IL-13, an important cytokine in the eosinophilic pathway. In a randomised trial, weekly administration of 180 or 360 mg of anti-IL-13 versus placebo for 16 weeks revealed a significant improvement in the endoscopy and histology score and in global assessment of disease severity, also in patients refractory to corticosteroids. This trial was extended open label for one year with a weekly 360 mg dose and showed continued improvement with mainly respiratory or nasopharyngeal infectious adverse effects in a minority of patients. This study supports the dominant role of IL-13 in eosinophilic pathogenesis.
Another novelty is Dupilumab, a monoclonal that inhibits both IL-4 and IL-13, thereby strongly impeding the Th2 inflammatory pathway. In a phase 2 control trial in 47 patients with active EoE dupilumab improved histology, endoscopy and dysphagia score and was generally well tolerated. Further studies are needed to help the clinician in his/her therapeutic selection between existing and forthcoming biologics, based upon efficacy, safety and cost-effectiveness.
Helicobacter pylori (HP) infection of the stomach remains clinically highly relevant, despite some waning interest in certain countries. That HP eradication counteracts the development of gastric adenocarcinoma was again nicely shown in a Swedish population-based cohort study following 95.176 individuals who had received HP eradication therapy between 2005–2012. Overall only 0.1% developed cancer and the risk decreased over time after eradication of the infection, stressing again the importance of HP infection as a major driver of gastric malignancy. Another important study evaluated the efficacy of HP eradication in individuals with a family history of gastric cancer in first-degree relatives. In 3.100 first degree relatives, HP eradication therapy was compared to placebo with a follow-up of 9.2 years. Gastric cancer incidence decreased from 2.7% in the placebo arm to 1.2% in the treatment arm and from 2.9% when infection persisted to 0.8% when infection was eradicated. Such findings again emphasize the importance of a thorough medical history taking, which should always include a detailed family history.
Remember the earlier published algorithm for second-line eradication treatment: – [bismuth quadruple or fluoroquinolone triple/quadruple] after failure with [PPI/amoxicillin/clarithromycin triple]; – [fluoroquinolone triple/quadruple] after failure with [bismuth quadruple]; – [bismuth quadruple or fluoroquinolone triple/quadruple] after failure with [non-bismuth quadruple therapy]? Should it not be better to recommend antimicrobial sensitivity testing after initial eradication failure? This question was studied in a randomised trial involving 382 patients, comparing susceptibility-guided therapy versus empiric bismuth quadruple therapy [14 days of esomeprazole 20 mg, bismuth 2 × 220 mg, 3 × amoxicillin 1000 mg, 3 × metronidazole 400 mg]. Per protocol eradication rates for susceptibility-guided versus empiric therapy were respectively 98% and 98% and per intention-to-treat 92% and 85% indicating that sensitivity testing was not superior to properly chosen empiric therapy. Are there other options in case of eradication failure in our current environment of increasing antimicrobial resistance? In a randomised study in 455 HP treatment-naive dyspeptica, 14 days of rifabutin-based triple therapy [omeprazole 120 mg, amoxicillin 3000 mg, rifabutin 150 mg] was compared to an active comparator [omeprazole 120 mg, amoxicillin 3.000 mg]. The intentionto- treat eradication rates, measured with the urea breath test, were respectively 84% versus 58%.
No rifabutin resistance was detected but diarrhea was induced in ~10%. The outcome was somewhat different in another retrospective study of 423 dyspeptics with failed prior eradication attempts, where 12 days of rifabutin triple [esomeprazole 40 mg, rifabutin 150 mg, amoxicillin 2 × 1000 mg] was compared to 10 days of quadruple with Pylera [capsules containing 140 mg bismuth subcitrate, 125 mg metronidazole, 125 mg tetracycline]. The cure rates with rifabutin triple versus Pylera quadruple were respectively 62% and 88%. It would appear that the efficacy for rifabutin-based therapy decreases according to previous therapy failures which does not occur for Pylera-based quadruple. It may well be that the future of HP eradication therapy will be simplified to dual ther0apy with a pCAB – amoxicillin combination. This was shown in a multicenter Japanese study of 335 infected treatment-naive individuals, comparing 7 days of dual therapy [vonoprazan 20 mg + amoxicillin 750 mg twice/day] versus triple therapy [vonoprazan 20 mg + amoxicillin 750 mg + clarithromycin 200 mg twice/day]. The eradication rates for dual versus triple for clarithromycin susceptible strains was respectively 86% vs. 95% and for clarithromycin resistant strains 92% vs. 76%. In the era of rising antimicrobial resistance, dual therapy with vonoprazan and amoxicillin may well become a new first-line therapy.
Figure 1.: Treatment Success at 1 Year (Intention-to-Treat analyses)
Small bowel diseases and infections – Gerhard Rogler (Switzerland)
Celiac Disease (CeD) is a complex immune-mediated gluten-sensitive enteropathy, occurring in ~1% of the population but 5 times higher in first-degree relatives of CeD patients. HLA haplotypes DQ2 and DQ8 are present in >90% and high-risk genotypes are more frequent in familial cases. Interestingly caesarean delivery and high ingestion of gluten in children with HLA risk alleles
during early childhood increases the incidence of CeD. Non-celiac gluten intolerance and/or non-celiac gluten sensitivity (NCGS) can affect up to 15% of the population. Enhanced anti-gluten IgG antibodies are also present in NCGS patients but the IgG subclass differs from CeD patients: more IgG1 and IgG3 in CeD and more IgG4 in NCGS. Besides the role of gluten, also amylase/trypsin inhibitors (ATIs), present in wheat and resistant to breakdown in the intestine, have been identified as indu-cers of innate immune responses via interaction with toll-like receptor 4 in NCGS. Sourdough fermentation of wheat degrades ATI tetramers as the pH drops below 4 due to activation of aspartic proteases, whilst in yeast fermentation, where pH remains above 4, ATI tetramers remain intact and may induce barrier damage. These novel findings have to be added to the symptom-generating high FODMAP content of wheat. Intestinal microbiota play a key role in modulating gut barrier integrity and immune/inflammatory responsiveness. Dysbiosis has been described in CeD patients, showing depletion of beneficial species such as lactobacillus and bifidobacteria and enrichment of potentially pro-inflammatory bacteria such as Veillonaceae etc. In general, a gluten-free diet reduces bacterial richness but changes in microbiol composition may differ. In healthy subjects, a gluten-free diet causes depletion of beneficial species such as Bifidobacteria and enrichment of oportunistic pathogens such as Enterobacteriaceae and E. coli whereas in CeD and NCGS patients, a gluten-free diet tends to re-equilibrate the microbiota populations by lowering the pro-inflammatory species. Undoutedly, the role of the microbiome is of fundamental importance in disease pathogenesis but we are just at the beginning of tackling its bewildering complexity, its composition determinants, its barrier-modulting effects, its toxins and its immune/inflammatory driving capabilities.
New population-based data have been published regarding longevity and ultimate outcome of CeD patients. A Finnish study evaluated 12.803 well documented CeD patients and found no increased mortality overall, nor from all-cause or gastrointestinal malignancy or cardiovascular disease, except for a 2.36 times higher mortality from lymphoproliferative diseases. Different results were obtained in a Swedish study involving 49.829 CeD patients followed for a median of 12.5 years. Compared to matched controls, there was a small but significant increase in mortality risk, overall cardiovascular, cancerous and respiratory. Assessment of the strictness of following a gluten-free diet in large cohort studies is almost impossible, yet a strictly followed life-long gluten-free diet may be a key variable as illustrated in a recent study where 16.776 Danish subjects with antibody-positive serum samples but without a formal CeD diagnosis were followed-up. Undiagnosed and subsequently untreated CeD was associated with an increased incidence of gastrointestinal, breast, uterus, head and neck cancer and of cardiovascular disease. These are shocking results which do stress the importance of making a proper diagnosis and careful follow-up of dietary instructions. Self-reported adherence rates to a gluten-free diet in CeD patients is usually high but unintentional gluten exposure may be more common than realized. Using novel antibodies that specifically detect gluten immunogenic peptides, exposure to gluten was detectable in over half the patients, stating full adherence to a gluten-free diet. How safe is occasional voluntary ingestion of gluten which was present in 8% of a recent CeD study? The mean estimated gluten amount was 185 g/year, consumed for an average of 9 years. Of these non-compliant patients, 75% reported no symptoms and only 23% had anti-transglutaminase IgA antibodies. The above studies show that there is a degree of overt but also of covert gluten intake in CeD patients following a gluten-free diet. Meticulously following a gluten-free diet life-long is difficult. That is the main driver for ongoing studies, looking at alternative possibilities beyond a gluten-free diet, shown in the figure below. (Figure 2.)
Gluteinases, such as latiglutenase can only break down minimal amounts of gluten. Zonulin inhibitors such as larazotide may help to tolerate inadvertent gluten intake. Whether blocking TG2 is helpful and safe is so far uncertain as TG2 is important in wound healing. This also holds for blocking HLA DQ2/8. Anti-Il-15 is being studied in refractory (pre-T cell lymphoma) CeD patients but several safety issues need to be considered. Studies with gluten vaccines, such as Nervax2 are also ongoing. Overall, the celiac dream of a normal, small intestinal mucosa while consuming gluten is still far from being achieved.
Most cases of microbial-related intestinal inflammation are caused by inadvertently consuming food that is contaminated with Campylobacter jejuni (the leading cause of foodborne bacterial enteritis), Salmonella, Escherichia coli or Listeria. In an important Danish, population-based cohort, patients were followed-up after a positive stool culture for Campylobacter concisus, Campylobacter jejuni, non-typhoidal Salmonella or negative culture. Interestingly, microscopic colitis developed in ~6% after C. concisus infection, compared to 0.6% after C. jejuni and 0.4% after salmonella. Earlier, mainly Australian studies had also pointed to a potential role of C. concisus in causing intestinal inflammatory disorders.
A newcomer is Covid-19 enteritis, where the virus enters the enterocytes after binding to the ACE-2 receptor, expressed in enterocytes and also in cholangiocytes. Gastrointestinal symptoms such as nausea, vomiting, watery diarrhea, and abdominal pain may occur in up to a third of infected patients and can occasionally precede the respiratory symptoms.
Gastrointestinal symptoms are common during and after chemotherapy. Out of 241 patients, ~20% reported chronic fecal incontinence and ~30% ongoing diarrhea, caused in ~50% by small intestinal bacterial overgrowth (SIBO) and in ~40% by not previously described bile acid malabsorption. SIBO has now also been found in some patients suffering from functional dyspepsia. In a randomised trial, 24 SIBO positive dyspeptics were treated for 2 months with placebo or 3 × 100 mg ursodeoxycholic acid (UDCA), shown in animal studies to have antibacterial and anti-inflammatory effects. UDCA caused a significant improvement of the dyspeptic complaints and of the lactulose-breath test results. SIBO is also common in patients with abdominal symptoms after bariatric surgery. Breath testing with glucose (75 g/250 ml) was positive in over 80% of about 100 such patients. A positive test was related to age, female gender and PPI use but not to the type of bariatric surgery. Improvement was obtained in ~60% after sequential gentamicin / metronidozole or metronidazole monotherapy.
The frequency of Clostridiodes Difficile (C.diff), a Gram-positive, spore-forming and toxin-producing anaerobe, is rising, in part related to antimicrobial use, old age and hospital admission. Medical therapy consists of vancomycin, fidaxomicin and less metronidazole and fecal microbiota transplantation for severe cases. Recently, oral vancomycin prophylaxis has been suggested and indeed a recent systematic literature review, involving 2.174 patients at risk, revealed a roughly 4 fold reduction in C.diff infection. Also, Saccharomyces boulardii has been explored in the prevention of hospital onset C.diff infection. Co-administered to hospitalized patients, prescribed antibiotics frequently linked to C.diff infection, reduced the risk from 0.82% to 0.56%. Finally, C.diff vaccination is being explored in healthy adults and was shown to be safe, well-tolerated and immunogenic, raising toxin A and B specific neutralising antibodies, and is going to be tested in phase III trials.
More than 30 million travelers are affected per year with Traveller’s Dairrhea (TD). Culture-independent diagnostic techniques have changed the approach to the diagnosis of enteric pathogens. Especially the use of high-throughput multiplex PCR panels has enabled better precision and more rapid results. However, latent infections or asymptomatic colonization cannot be distinguished from active, clinically-significant infections and mixed infections are more often identified compared to using routine culture technology. Such a multiplex PCR panel (covering 13 enteric bacteria, 4 protozoan parasites and 5 viruses) was positive in 75% of symptomatic and in 57% of asymptomatic travellers. Enteroaggregative E. coli was present in 40%, enterotoxigenic E.coli in 34% and enteropathogenic E.coli in 32%. The only pathogens significantly associated with diarrhea were enteropathogenic and enterotoxigenic E.coli. Protozoan parasites were not detected. Such results challenge the widespread use of multiplex-PCR approaches as so far only diarrheagenic E.coli seem related to symptomatology. Increasingly, multidrug resistance, including resistance to fluoroquinolones and third-generation cephalosporins is observed in E.coli strains causing traveller’s diarrhea. Over 50% of travellers returning from Southeast Asia, and close to 50% returning from Northern Africa are colonized with multidrug-resistant Enterobacterales. All this provides further evidence for the need to restrict the use of antimicrobial agents and the need for continuous monitioring of multidrug resistance.
Dietary emusifiers and other food additives such as nanoparticles have been associated with intestinal disease. The European Food Safety Agency (EFSA) regulates the amount of such additives, but methodologies to measure the actual food content and overall consumption are suboptimal. Potential pathogenic mechanisms associated with food additives are illustrated in the figure below. (Figure 3.)
Emulsifiers and nanoparticles have been shown to have effects on gut microbiota, mucosal barrier and inflammatory pathways. Silicon dioxide and titanium dioxide accumulates in human tissues, especially jejunum and ileum. We do not know at present the exact consequences of the numerous food additives, but in all probability they will turn out to be non-negligeable contributors to pathophysiology and disease.
Figure 2.: Therapeuticoptions for CeD: What else beyond gluten-free diet?
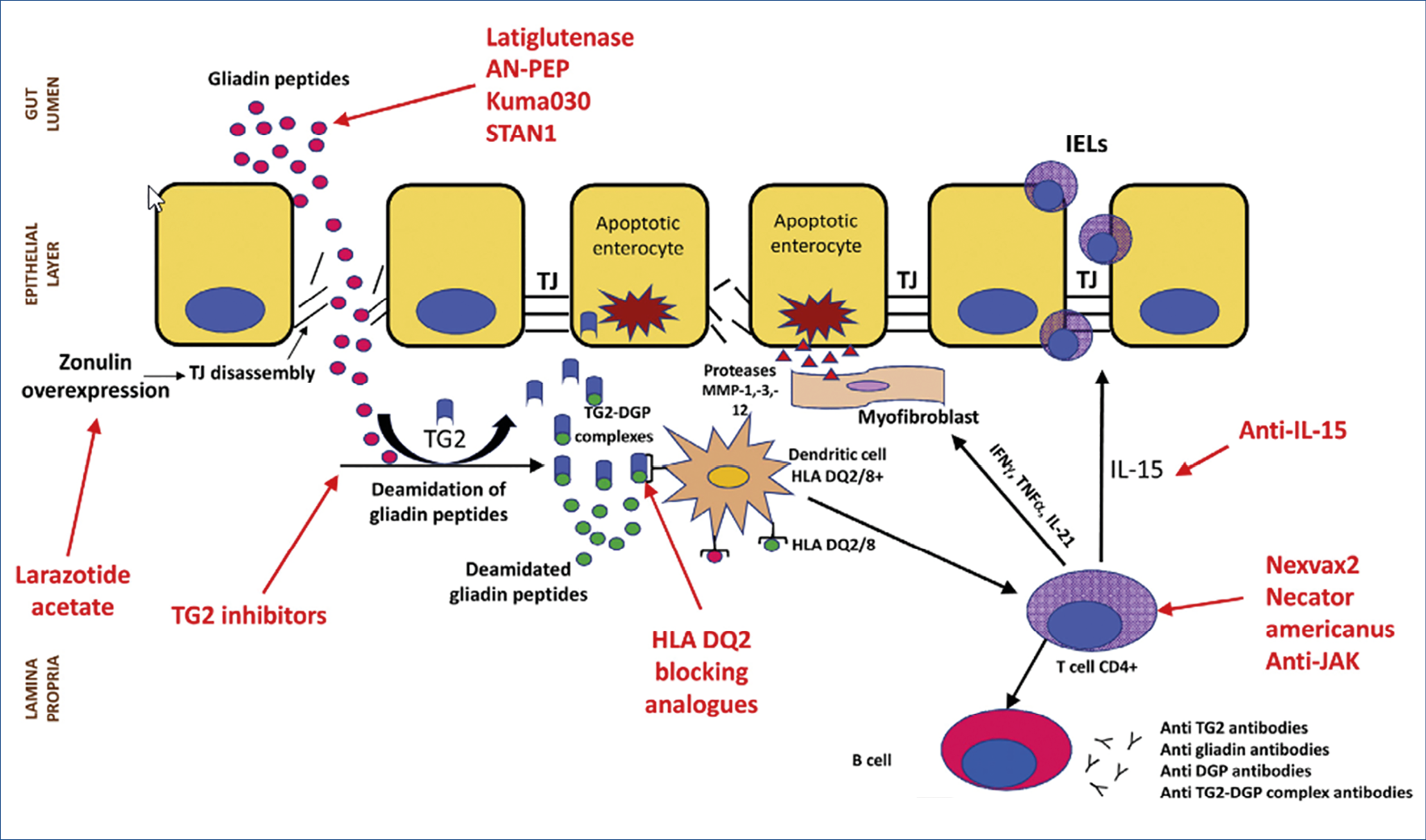
Figure 3.: Potential pathogenic mechanisms associated with food additives
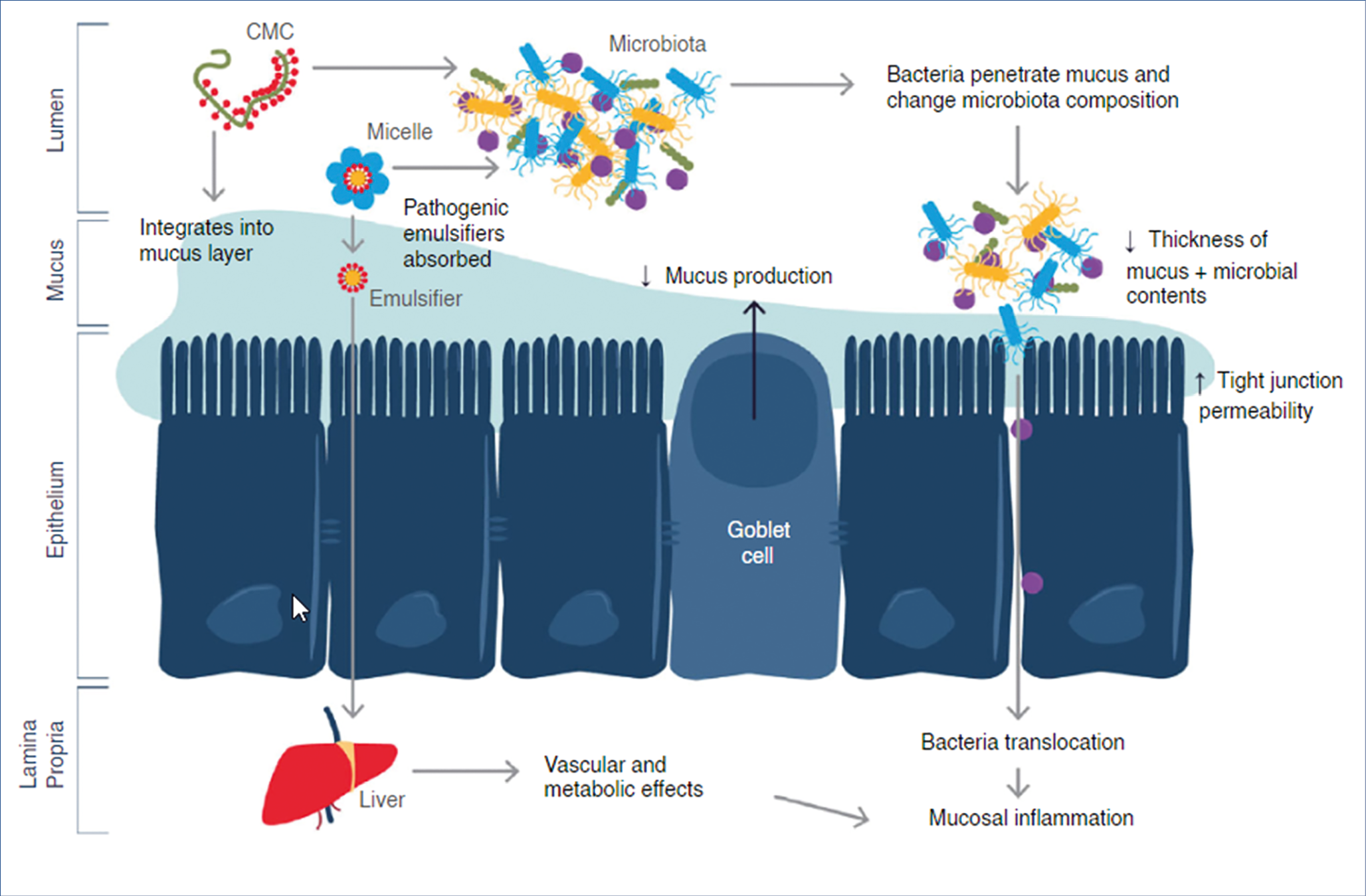
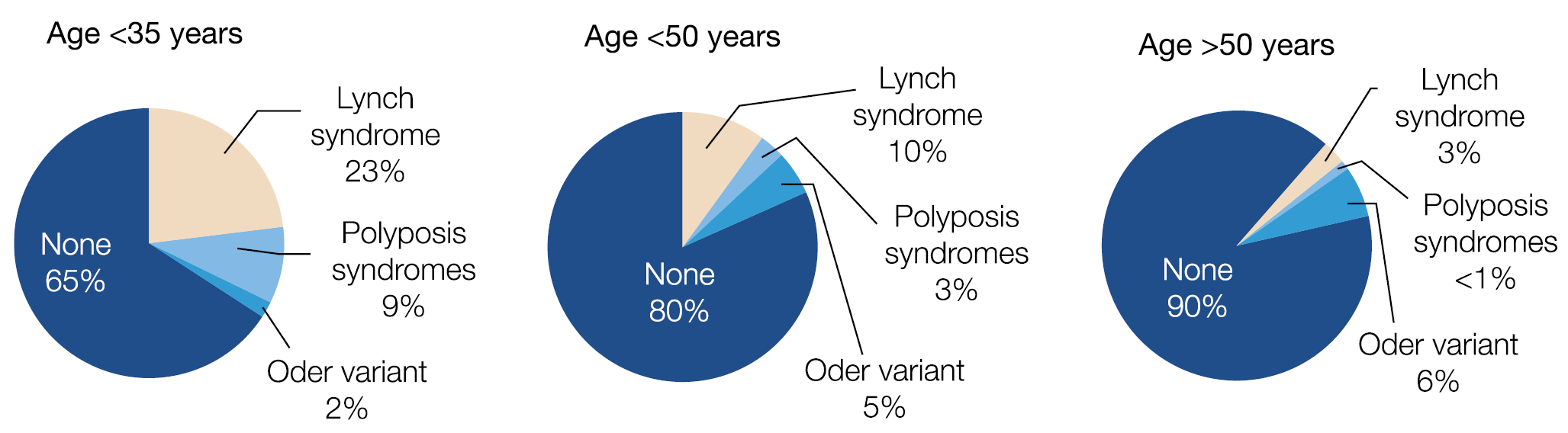
Figure 4.: Genetic predisposition is different in young vs. older
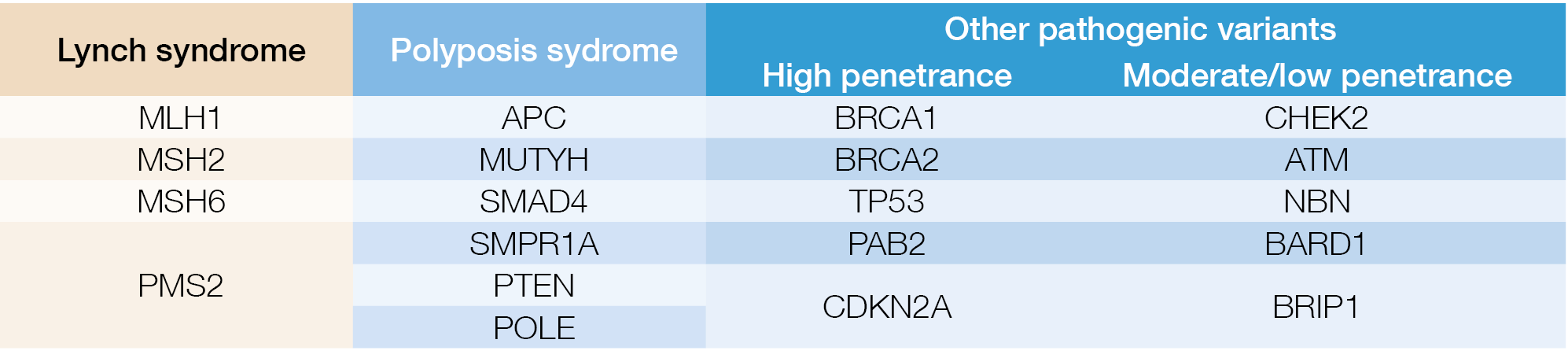
Novel large bowel developments – Jaroslaw Regula (Poland)
One in 10 new colorectal cancers (CRC) occurs now in individuals younger than 50 years, which is a doubling of the incidence since the early 1990s and 3 out of 4 do not have a family history. Colonic and rectal cancer is estimated to rise to respectively 11% and 23% by 2030 in <50 years old individuals. Why is there a predominance of distal, especially rectal cancer? Can this age change be related to environmental factors as suggested by the high incidence in the Mississippi Delta and Appalachia regions, known for poverty, unemployment, poor access to health care and perhaps high exposure to agricultural toxins and fertilisers? Or does the genetic predisposition differ according to age category as suggested in the figure below. (Figure 4.) Or, are there changes in the microbiome as a key driver for colonic cancer? A mega-analysis of close to 30.000 British CRC patients, matched with 137.000 controls revealed a dose-dependent effect of oral antibiotic use, mainly targeting anaerobes, in raising the risk of colonic but not rectal cancer. All these alarming findings stress the urgent need for new risk assessment algorithms, including family history, BMI, smoking, alcohol, physical activity, fasting glucose, red meat and vegetable consumption, folate, NSAID and antimicrobial use, etc. combined with acompilation of genetic factors.
Evaluation of such risk predictive models is ongoing and will need to be applied, also in Europe. Help is coming for the overburdened pathologists as artificial intelligence with deep learning modalities can detect microsatellite instability and mismatch-repair deficiency in standard hematoxylin-eosin stained slides with 95% sensitivity and 67% specificity. Almost all European countries have embarked on CRC screening and surveillance, many with FIT as a selector for colonoscopy or with straight colonoscopy. The problem of interval cancer is not solved and how far the interval between colonoscopies can be extended is still uncertain. Obviously, the quality of the colonoscopy is of paramount importance. Characteristics of a high-quality colonoscopy is based upon high cecal intubation rate, excellent bowel preparation and colonoscopist experience, expressed by high adenoma detection rate >20%. The figure below illustrates the impact of colonoscopy quality for interval cancer incidence ratio’s, closely resembling the not shown mortality ratio’s. (Figure 5.)
A single negative, high quality screening colonoscopy provides an incidence and mortality reduction lasting up to 17.4 years. High quality versus low quality examination reduces the CRC incidence from 74% to 21% and the mortality from 85% to 60%. Elimination of low quality colonoscopy should be of top priority.
Chronic mesenteric ischemia is a rare but severe incapacitating disease that is under-appreciated and under-treated in clinical pratice. European guidelines were recently published in the UEG Journal (2020; 8: 371.). Classical symptoms are – postprandial abdominal pain 10-30 min after meals, lasting 1-2 hours; – permanent abdominal pain aggravated by eating; – weight loss; – fear of eating; – potentially progressing to often fatal acute intestinal infarction.
The basic pathophysiological disturbance responsible for the pain is the impossibility to raise the mesenteric blood flow by 30-100% after meals to comply with the increased oxygen demand. Leading causes of ischemia are – atherosclerotic vascular stenosis; – median arcuate ligament compression of the celiac artery; – mesenteric venous thrombosis; – vasculitis; – non-occlusive mesenteric ischemia, related to insufficient mesenteric microcirculation in patients with cardiac failure, pulmonary hypertension, severe anemia and vasospastic conditions. Helpful diagnostic tests in case of a suspicious history are upper gastrointestinal endoscopy, duplex ultrasound, CT/MRI – angiography, contrast angiography etc. A newcomer in this field is the per-endoscopy use of a visible light-spectroscopy catheter, measuring mucosal oxygen saturation in the antrum, bulb and descending duodenum. Therapy consists of surgical revascularisation or percutaneous mesenteric artery stenting of transluminal angioplasty.
Figure 5.: Significance of quality assurance in colonoscopy
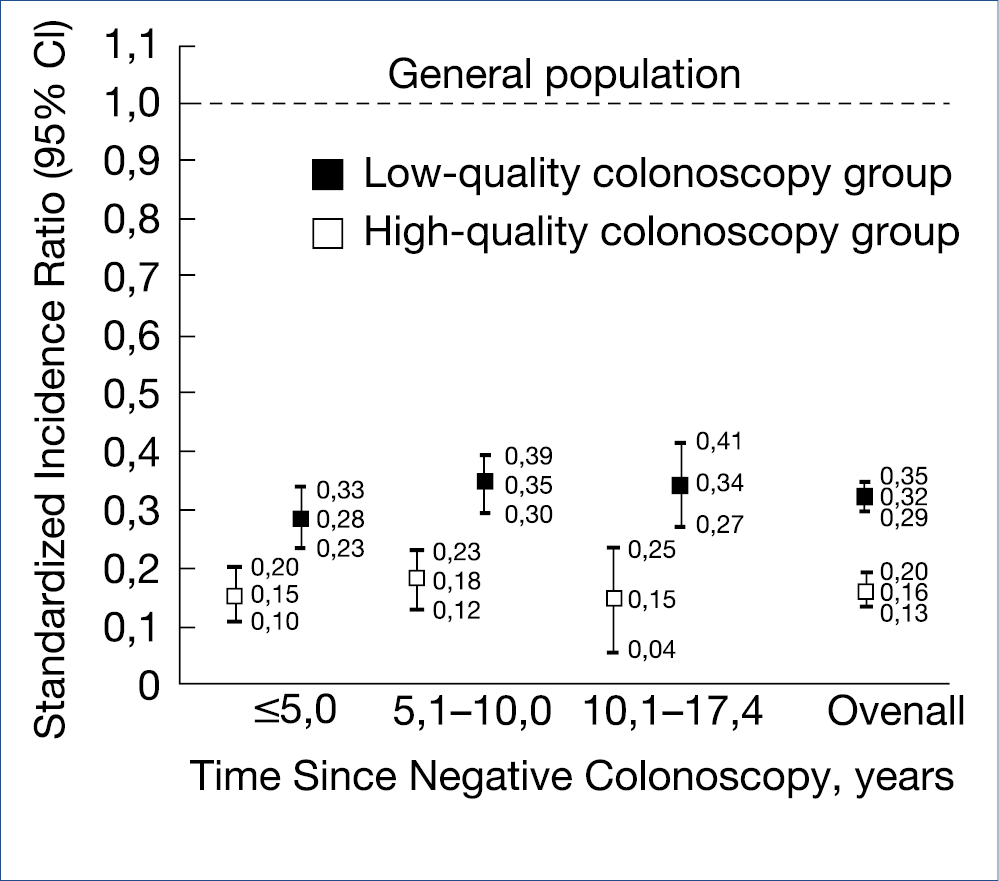
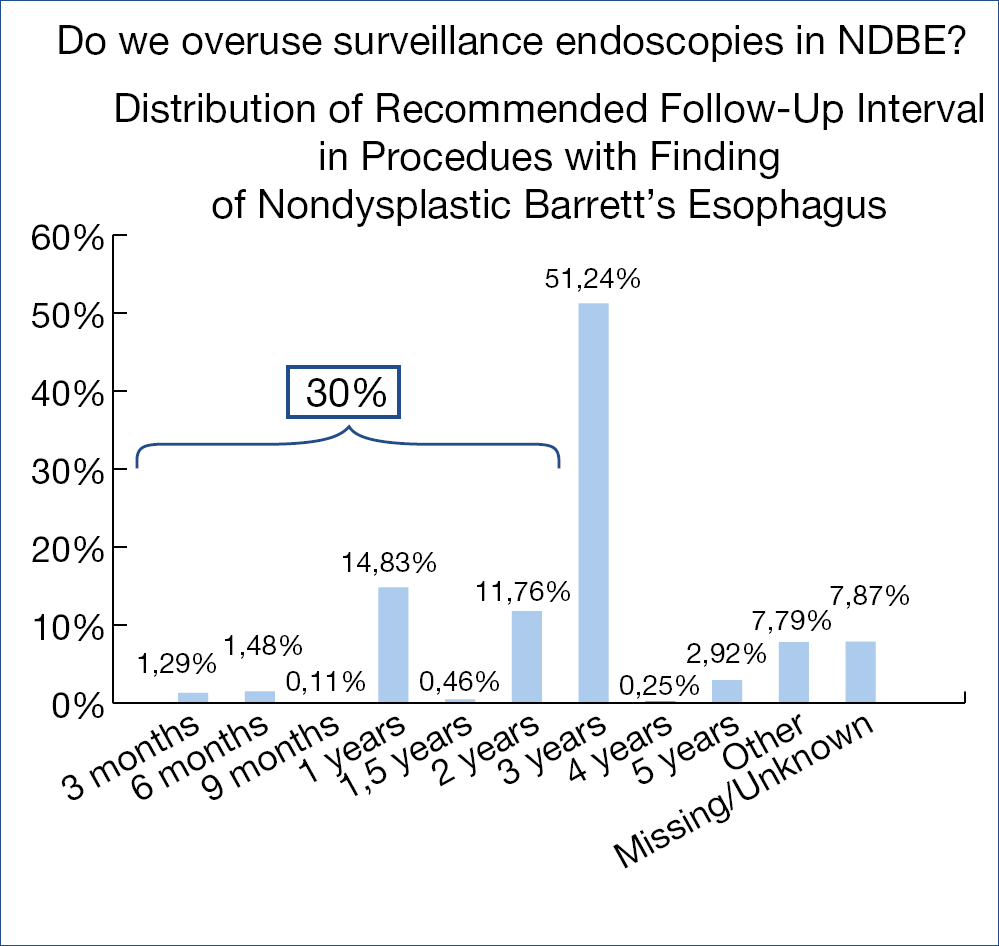
Figure 6.: Recommended surveillance in 25,945 patients with NDBE, NDBE, non-dysplastic Barrett’s esophagusNovel developments in upper intestinal endoscopy – Oliver Pech (Germany)
A systematic review of multi-national studies collected 10.632 patients with esophageal columnar metaplasia (Barrett esophagus/BE) detected during the first index endoscopy for reflux symptoms. This cohort consisted of 80% male gender, mean age 59 years and average BE length of 3.5 cm. The prevalence of low grade (LG)-dysplasia was 10% and of neoplasia (combining HG-dysplasia and adenocarcinoma) was 7%, varying from 4-10%. The authors suggest that a neoplasia detection rate of at least 4% could be used as an endoscopy quality indicator (comparable to the adenoma detection rate in the colon).
Is there over-utilization of surveillance endoscopy in BE patients without dysplasia (NDBE)? Guidelines recommend surveillance endoscopy every 3-5 years in case of NDBE. Endoscopy data of close to 800.000 patients from an American GI Quality improvement consortium registry were analysed: close to 60.000 (some 7.5%) were related to BE. Mean BE length was 2.3 cm, 86% non-dysplastic, 6% indefinite/low grade and 1.5% high grade dysplasia and ~5% missing data. The figure below shows the stunning findings. (Figure 6.)
In 30% there was non-compliance with the guidelines; several reasons including financial incentives may explain the shift to early surveillance. It is perhaps wise at this point to refresh the European surveillance guidelines for NDBE: – irregular Z-line/BE <1 cm: no surveillance; – maximum extent <3 cm: 5 years; – maximum extent <10 cm: 3 years; maximum extent >10 cm: referral to expert center; – beyond age 75 years and no prior dysplasia: no further surveillance.
Many studies have shown that (pre)-malignant lesions are often subtle and difficult to detect during endoscopy. In the upper intestinal tract, up to 25% of mainly early cancers are missed (BE cancers 25%; gastric cancers at least 10%). Could real-time artificial intelligence, either machine learning or artificial neural networking, help in the detection of upper gastrointestinal cancer? A gastrointestinal artificial intelligence diagnostic system was developed based upon ~1 million endoscopic images from over 80.000 chinese patients. The diagnostic performance was equal to that of expert endoscopists. Artificial intelligence using deep neural networking was also evaluated in the prediction of the infiltration depth of esophageal squamous cancer. Overall accuracy was 91% for artificial intelligence compared to 89.6% for expert endoscopists and was shown to reliably differentiate cancer up to submucosal level 1 (which can be curatively treated with submucosal dissection) from deeper infiltrating malignancy.
Artificial intelligence has also been evaluated for quality improvement of upper intestinal endoscopy, where often full systematic mapping of the entire stomach surface is not obtained, by monitoring the presence of blind spots. The system was based upon deep convolutional neural networking and deep reinforcement learning and used to monitor the blind spots in 324 Chinese patient videos, compared to the analysis by expert endoscopists. The blind spot rate dropped significantly from 22% to 6%, helping to create a more complete photodocumentation report with limited inspection time lengthening from 4.24 min to 5.03 min. It is to be expected that artificial intelligence – based platforms, of which many are in development, will be incorporated in all fields of endoscopy, improving the overall quality, particularly of the less-experienced endoscopist.
Peroral endoscopic pyloromyotomy (G-POEM) has been added to pyloric balloon dilation and Botox injection to the endoscopic therapeutic treatment of severe gastroparesis. The procedure is illustrated in the figure below. (Figure 7.)
Ten studies involving 281 patients were meta-analysed with the [gastroparesis cardinal symptom index] and the [4 hour solid-phase gastric emptying scintigraphic test]. Overall G-POEM was clinically successful, improving gastroparesis symptoms and scintigraphic gastric emptying in these early follow-up studies. The complication rate was limited mainly related to bleeding, pneumoperitoneum and abdominal pain. More evaluations are needed to select the best responders and to clarify the long-term efficacy.
Mortality of acute esophagogastric variceal bleeding varies around 20% at 6 weeks after onset. Data from bleeding registries show that a significant proportion of such patients have a delay of > 24 hours before expert endoscopy was carried out. Is early application, within 2 hours of onset, of hemostatic powder (hemospray) useful as a bridge to more definite endoscopic therapy, carried out within 24 hours after admission, in more stabilized patients? A randomized trial involving 86 Egyptian patients were studied using a combined primary end point of endoscopic hemostasis and clinical hemostasis. The primary endpoint was not achieved in 5/43 of the hemospray group compared to 12/43 of the control group. Hemospray could be considered as a bridge to definitive treatment with band ligation or injection of cyanoacrylate for gastric varices.
Several studies have evaluated the impact of direct oral anticoagulants (DOACs), which are increasingly prescribed, on bleeding risk related to endoscopy. What follows is a summary of the take-home messages from those studies: – risk of intraprocedural bleeding may be as high as ~5% in low-risk endoscopies with biopsies in patients who did not omit the DOAC morning dose; – delayed bleeding does occur more often when DOAC treatment is resumed earlier than recommended in the European guidelines; – routine peri-endoscopic bridging with heparin substantially increases the risk of bleeding without providing any clinical benefit; – short-term interruption of anticoagulation, as recommended by the European guidelines, appears to be a safe and effective strategy for containing bleeding events and minimising thromboembolic risk.
Figure 7.: G-POEM – The procedure
Novel developments in biliopancreatic endoscopy – Marco Bruno (Netherlands)
Prevention of post-ERCP pancreatitis remains of major concern; definite risk factors are: young age, female gender, suspected Oddi sphincter dysfunction, history of recurrent pancreatitis or previous post-ERCP pancreatitis, and absence of chronic pancreatitis. Preventive measures include: – rectal non-steroidal anti-inflammatory drugs (NSAIDs) such as 100 mg indometacin or diclofenac; – protective short 5-Fr pancreatic duct stent; – peri-procedural Ringer-hyperhydration. Comparing dose escalation from 100 mg to 200 mg indometacin was evaluated in a randomized trial involving over 1.000 patients. Indometacin dose escalation did not confer any advantage and the adverse events were also similar. Current practice should therefore be continued according to the 2014 European guidelines.
Despite a multidisciplinary approach, distinguishing between benign and malignant bile duct strictures remains challenging. Pooled literature data of brush cytology show a sensitivity and specificity of diagnosing malignancy of respectively 45% and 99%, and of targeted intraductular biopsy of 48% and 99%. An overview of various combination modalities is shown in the figure below. (Table 1.)
Can detection of genomic alterations in ERCP-obtained biliary specimens be of diagnostic help in detecting malignancy? A 28-gene ‘next Gene Sequencing panel’ (BiliSeq) was evaluated in 252 patients with indeterminate bile duct strictures. The sensitivity and specificity of BiliSeq for malignant strictures was respectively 73% and 100%. In comparison, sensitivity and specificity for elevated serum Ca19.9 and abnormal histopathology were respectively 76% & 48% and 69% & 99%.
The combination of BiliSeq and histopathology increased the sensitivity to 83% and maintained a specificity of 99%. BiliSeq improved the sensitivity of histopathology from 35% to 77% for biliary brushings and from 52% to 83% for biliary biopsies. Among patients with primary sclerosing cholangitis, BiliSeq had a sensitivity of 83% compared to 8% for histopathology. Besides improved diagnostic capability, genetic analysis may also have therapeutic consequences, shown by 2 patients with ERBB2-amplified cholangiocarcinoma, receiving a trastuzumab-based regimen. All these genomic developments are promising and one may even speculate whether examination of bile collected from the duodenal lumen would be an appropriate substrate for such genetic determinations.
A recurring theme relates to the need for urgent ERCP within 24 hours after admission in predicted severe biliary pancreatitis. Guidelines recommend that: – there is no indication for urgent ERCP in predicted mild pancreatitis; – that urgent ERCP should be performed in patients with gallstone pancreatitis with concomitant cholangitis; and suggest that ERCP might be beneficial in patients with cholestasis but without cholangitis. It is unclear whether urgent ERCP is beneficial in patients with gallstone pancreatitis without cholangitis and without significant cholestasis although this is commonly done in clinical practice. Unfortunately, almost all previous randomised studies had important shortcomings. A recent study re-evaluated urgent ERCP with sphincterotomy in patients with predicted severe acute gallstone pancreatitis. Out of a large group of well over 1.000 acute pancreatitis patients, 232 were randomized to urgent ERCP+sphinterotomy versus conservative treatment. The primary endpoint of combined mortality or major complication dropped from 43% in the conservative group to 32% in the treatment arm in patients with cholestasis whereas the results were the same in patients without cholestasis. The main difference was observed in patients presenting with acute cholangitis. The authors stress that the only indication for urgent ERCP and sphincterotomy is acute biliary pancreatitis (regardless of predicted severity or presence of cholestasis) AND cholangitis (characterized by high fever with/without chills, presence of common bile duct stones on imaging or dilated common bile duct or progressive cholestasis for at least 2 consecutive days). Without concomitant cholangitis, the patient can be observed and the ERCP postponed until the pancreatitis attack has subsided.
Very high risk patients with acute cholecystitis may be unfit for (laparoscopic) cholecystectomy. Percutaneous cholecystostomy is usually performed, followed by delayed cholecystectomy once the patient’s overall condition has improved.
However, many elderly patients suffer from multiple co-morbidities that render them unfit for surgery. Current guidelines do not provide definite recommendations on how to manage such patients. If sepsis cannot be controlled, the gallbladder is usually drained percutaneously, but the optimal management after drainage without the option of cholecystectomy remains undefined. Recently EUS-guided gallbladder drainage has gained attention as a method of internally draining the gallbladder and removing gallstones in high-risk patients. The endoscopic methodology and necessary equipment is comparable to that used for EUS-guided pancreatic cyst/abscess drainage. Several comparative studies have suggested that EUS-guided drainage was associated with improved outcomes comparted to percutaneous drainage.
Now, also a randomized comparison of EUS-guided versus percutaneous gallbladder drainage has been published. The EUS-guided approach was superior with respect to 30-day and 1-year adverse event rates, recurrent cholecystitis and need for reintervention. Predictors for recurrent acute cholecystitis were age >75; male gender; Charlson comorbidity index >5; severity of initial cholecystitis and prior treatment with percutaneous drainage. This study therefore adds high level evidence that EUS-guided drainage is to be preferred for unsuitable candidates for cholecystectomy. As the need for such advanced endoscopic procedures will rise, also non-tertiary regional hospitals will have to be involved through facilitating gaining expertise by centralizing patients within the GI team.
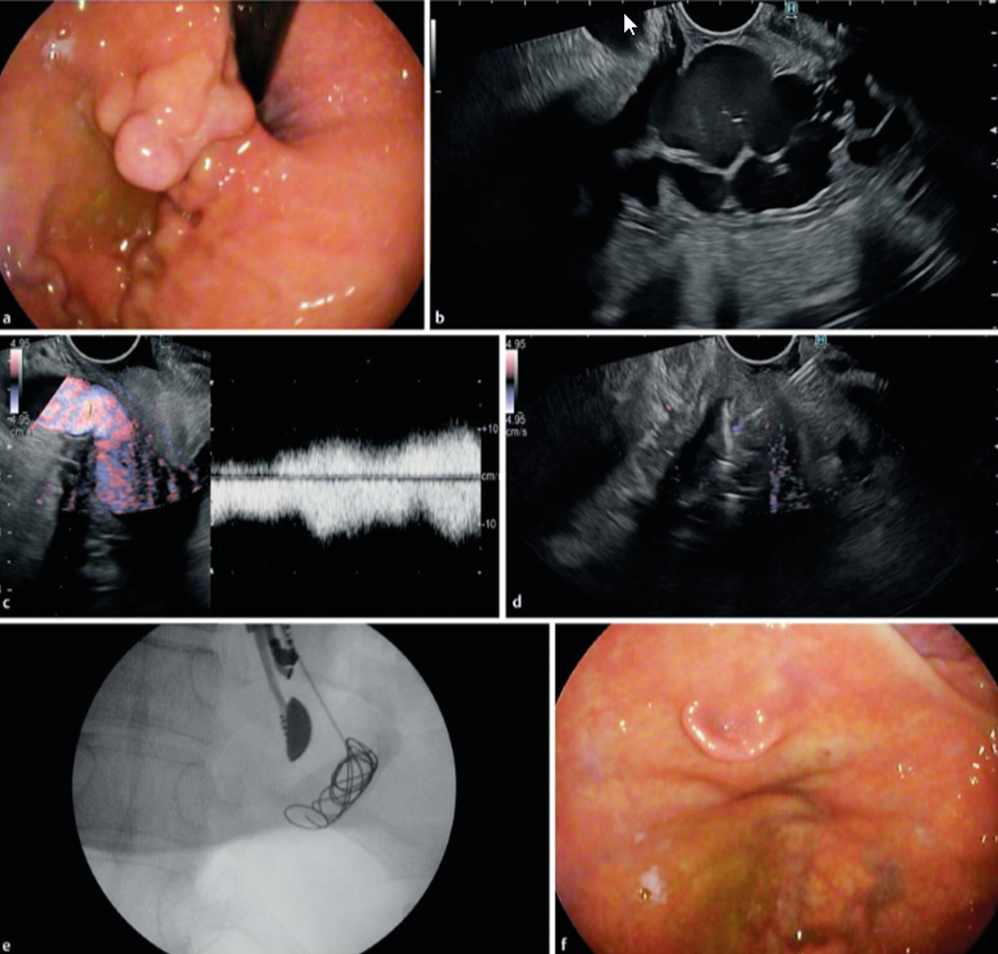
Bleeding gastric varices remains a nightmare, usually treated by intravariceal injection of cyanoacrylate with / without additional coils. In a recent study, EUS-guided coil embolization & cyanoacrylate injection of gastric varices was compaired to coiling alone in a controlled trial involving 60 patients. The technique is illustrated in the figure below. (Figure 8.)
Metal microcoils, up to 0.035 inches and covered with synthetic fibers promote clot aggregation and serve as a scaffold for glue adherence. Technical success and complete obliteration was achieved in almost all patients, but more coils were used in the coiling only arm. The combination of coils and cyanoacrylate was superior in the immediate disappearance of varices, varix re-appearance rate, rebleeding rate, and reintervention rate. Thus EUS-guided intervention to treat bleeding gastric varices is a valuable technique to complement standard endoscopic therapy and / or emergency TIPS placement. EUS-guided coil embolization combined with cyanoacrylate injection is superior to coil embolization alone because of lower rate of rebleeding and reintervention
Table 1.: Diagnosis of Indeterminate Bile Duct Strictures
State of the Art
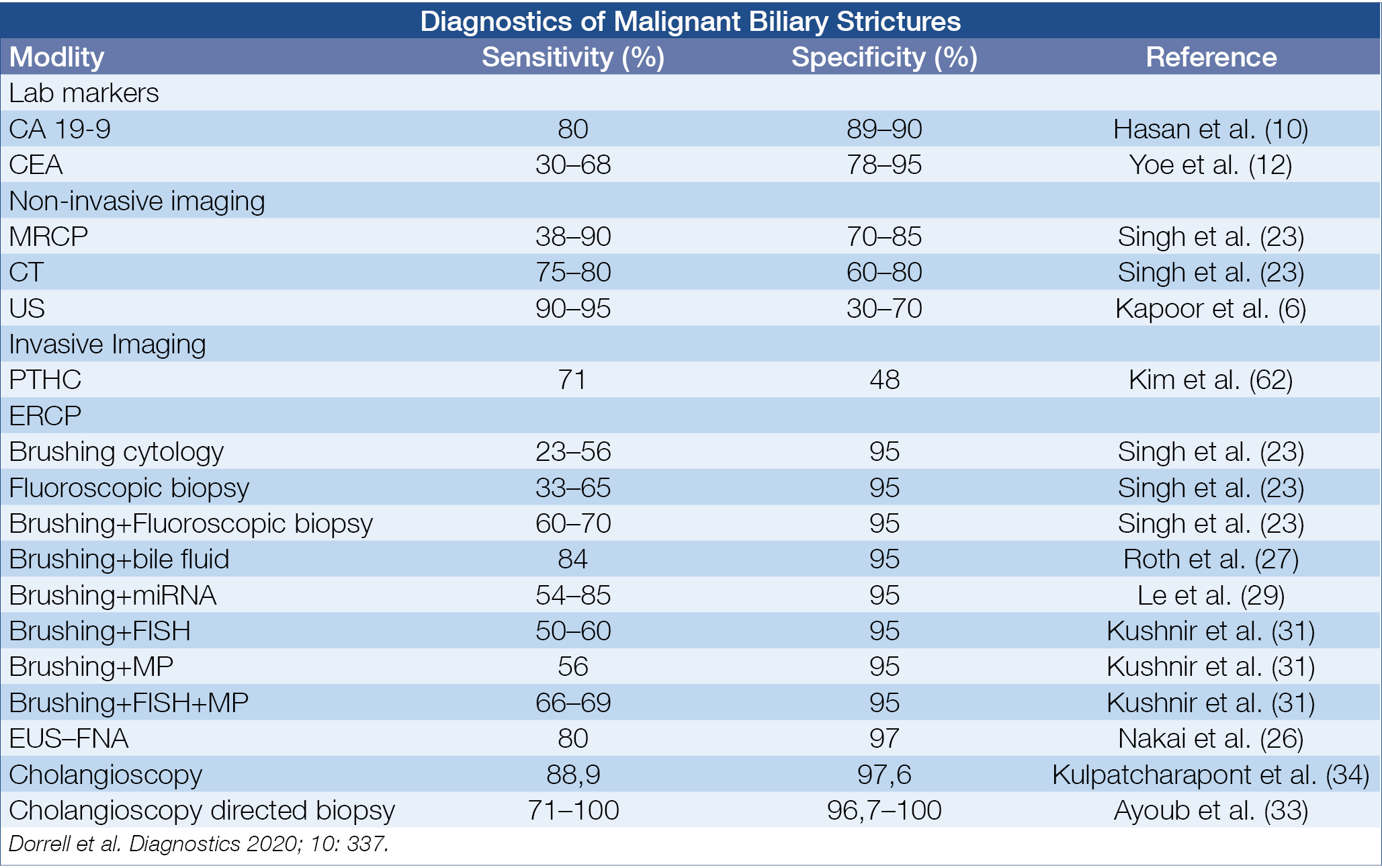
Figure 8.: EUS-guided Coil Embolization & Cyanoacrylate Injection in Gastric Varices versus Coiling alone

Novel developments in biliopancreatic endoscopy – Marco Bruno (Netherlands)
To find out how much surveillance colonoscopy is needed should essentially been based on the colorectal cancer (CRC) risk after adenomatous and serrated polyp removal. The cumulative incidence of CRC after index polyp removal was analysed in 3 large cohorts with a median follow-up of 10 years as illustrated in the figure below. (Figure 9.)
Patients bearing an advanced adenoma or large serrated polyp at initial endoscopy had a manifestly higher risk for later CRC. Comparable results were also published from a Californian cohort of over 60.000 patients followed-up for ~8 years, also indicating that the CRC risk is highest for patients with advanced adenoma or large serrated polyp. To find out whether the risk profile of adenomatous polyps can be further refined, Polish investigators analysed the data of the large scale Polish CRC screening program involving 236.089 patients followed for a median of ~7 years after index adenoma removal.
A comparison was made between traditional advanced adenoma [>10 mm, or HG-dysplasia, or >3, or villous], present in some 40% of their cohort, compared to novel high-risk characteristics [>20 mm or HG-dysplasia] present in some 10% of the adenoma cohort. The cumulative CRC risk was especially high in the latter high-risk patients, reaching a value of 2% after some 10 years. The take-home messages were – that CRC risk is not increased in case of low-risk adenomatous / serrated polyp in contrast with the increased risk for high-risk adenoma, especially when the novel criteria are used and – that 1 surveillance endoscopy already leads to a ~50% risk reduction, irrespective of the baseline polyp characteristics.
Polyp removal via snare polypectomy of endoscopic mucosal resection (EMR) is not without shortcomings: on average, resection is incomplete in about 10% but up to 30% after piecemeal EMR and there is a risk of delayed bleeding in 5-10% after large EMR. Incomplete polyp resection is considered to be responsible for at least a quarter of the so-called interval CRCs. The usefulness of additional coagulation of the mucosal defect margin was evaluated in a controlled trial, as illustrated in the figure below showing a substantial reduction of the recurrence rate. (Figure 10.)
The efficacy of clipping the defect to decrease the risk of bleeding after removal of large laterally spreading lesions was evaluated in 919 lesions, larger than 20 mm. Complete clip closure of the defect was possible in 68% with a median of 4 clips. The risk of post-procedure bleeding decreased overall from 7.1% to 3.5% and was especially obvious for proximal polyps, decreasing the bleeding rate from 9.6% to 3.3%. Similar results were obtained in another trial evaluating clipping in 235 large laterally spreading lesions. Delayed bleeding decreased overall from 12.1% to 5.1% but to 1.5% when complete closure of the defect was possible in over half the patients. A meta-analysis, involving 7.197 large polyps, treated by EMR, comparing clipping versus control showed a decrease of post-procedure bleeding from 10% to 3.8% for large and proximal lesions, but no significant effect for small and distal polyps. Prophylactic clipping is therefore only indicated for proximal large spreading lesions leading to a 60% reduction in delayed bleeding.
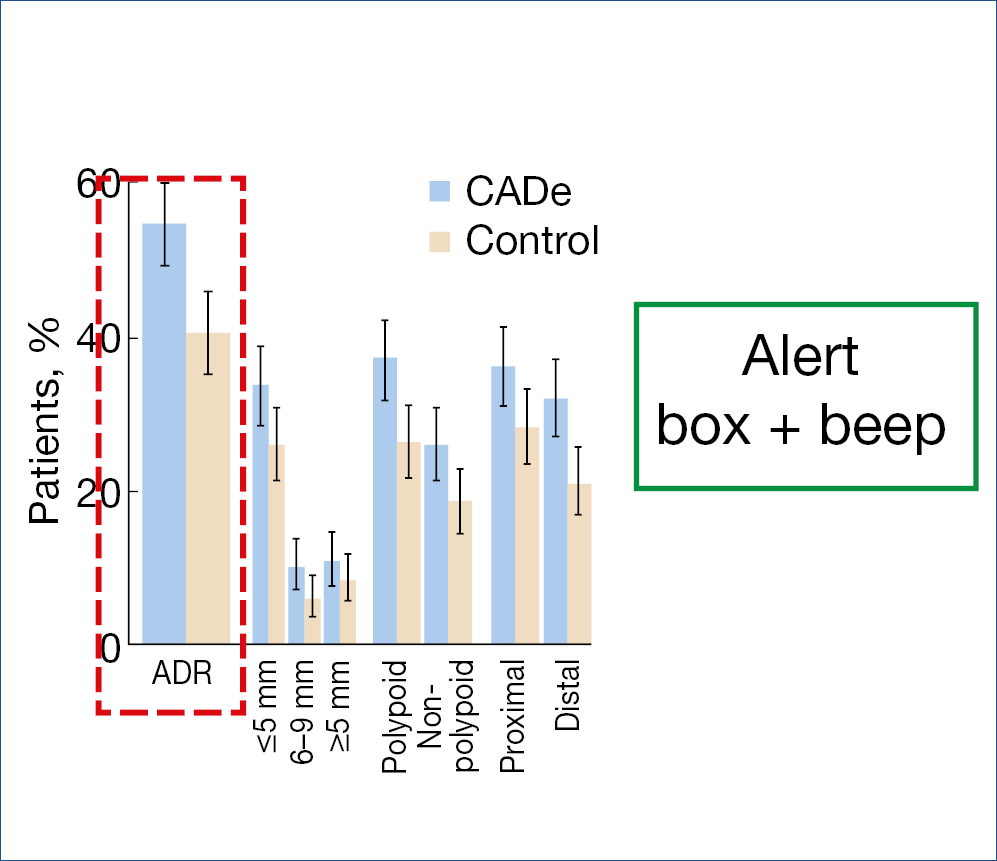
Artificial intelligence also entered the field of colonic endoscopy. Several systems are being developed and evaluated. The CADe system, alerting the endoscopist by drawing a box around a suspicious polyp together with a beep signal, was evaluated in over 1.000 patients. The polyp detection rate in the computer-aided detection group was 52% versus 37% in the sham group. The corresponding adenoma detection rates were 34% and 28% and adenomas detected per colonoscopy were 0.58 vs. 0.38, a 1.53 fold change. Another study shown below gave comparable results. (Figure 11.)
A meta-analysis, comparing the CADe system versus control in over 4.000 patients resulted in a pooled adenoma detection rate of 36.6% vs. 25.2%.
The ENDOANGEL system was evaluated in ~700 patients and compared to controls; the adenoma detection rate was 16% with the ENDOANGEL-assisted colonoscopy versus 8% in the control group. The AGC (automated quality control) system was evaluated in 659 patients and revealed an adenoma detection rate of 28.9% versus 16.5% in the control arm. It is obvious from all those studies that artificial intelligence will become established in helping the endoscopist in polyp detection and automated evaluation of procedural quality.
There is still some controversy regarding the optimal timing of endoscopy for acute lower gastrointestinal bleeding. In a randomized study, early (<24 hours) was compared to elective (24-96 hours) colonoscopy in 170 patients with –hematochesia (× 3/8 h), – requiring transfusion or shock. No significant differences were found with respect to detection of stigmata of recent hemorrhage, rebleeding rate and transfusion requirement. Similar findings were obtained in a recent meta-analysis, confirming that elective colonoscopy between 24 and 96 hours does not increase the risk of rebleeding, the need for transfusion, the finding of stigmata and does not increase all cause mortality.
Figure 9.: Risk of CRC after adenoma and serrated polyp removal
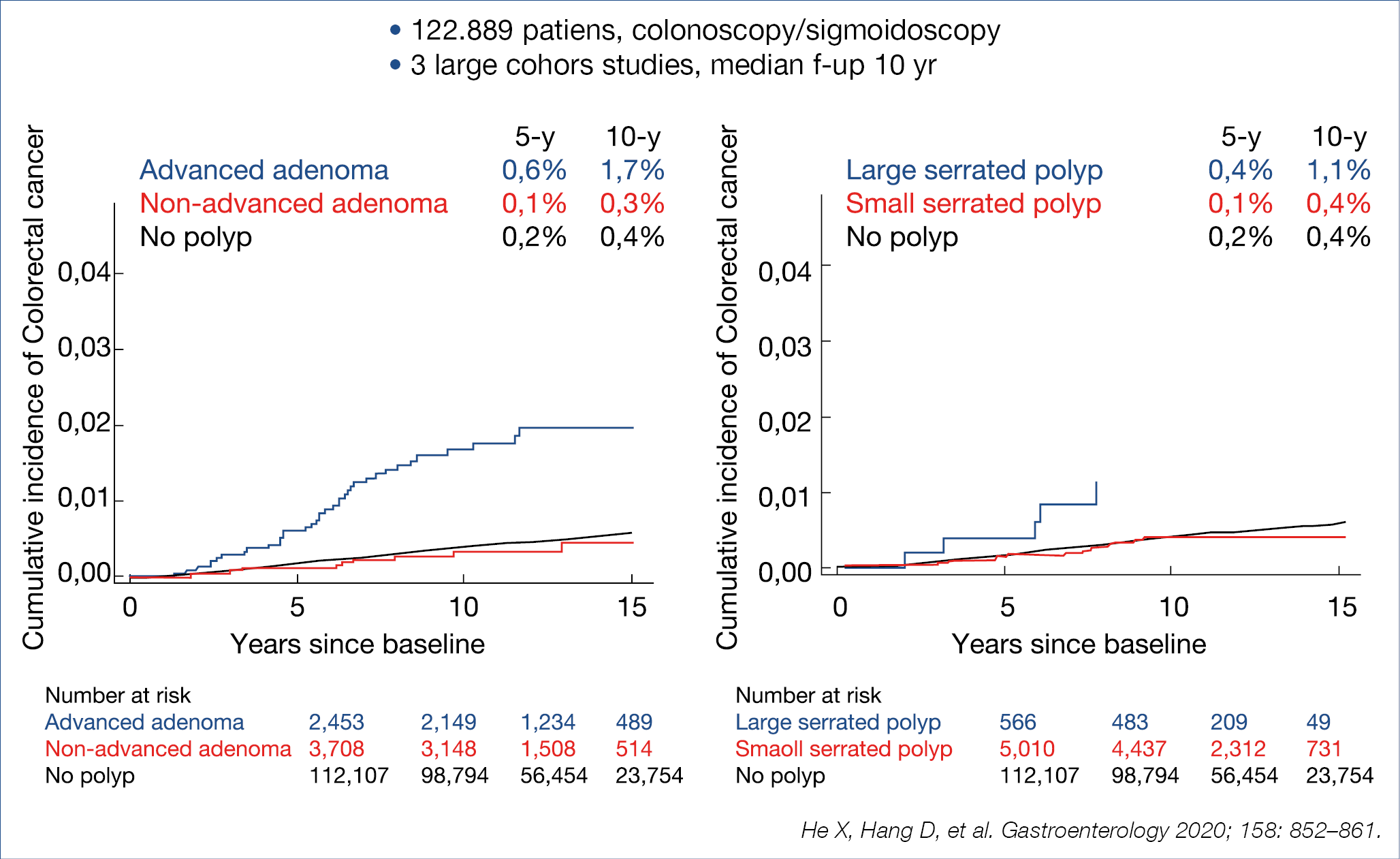
Figure 10.: Thermal ablation of mucosal defect margin
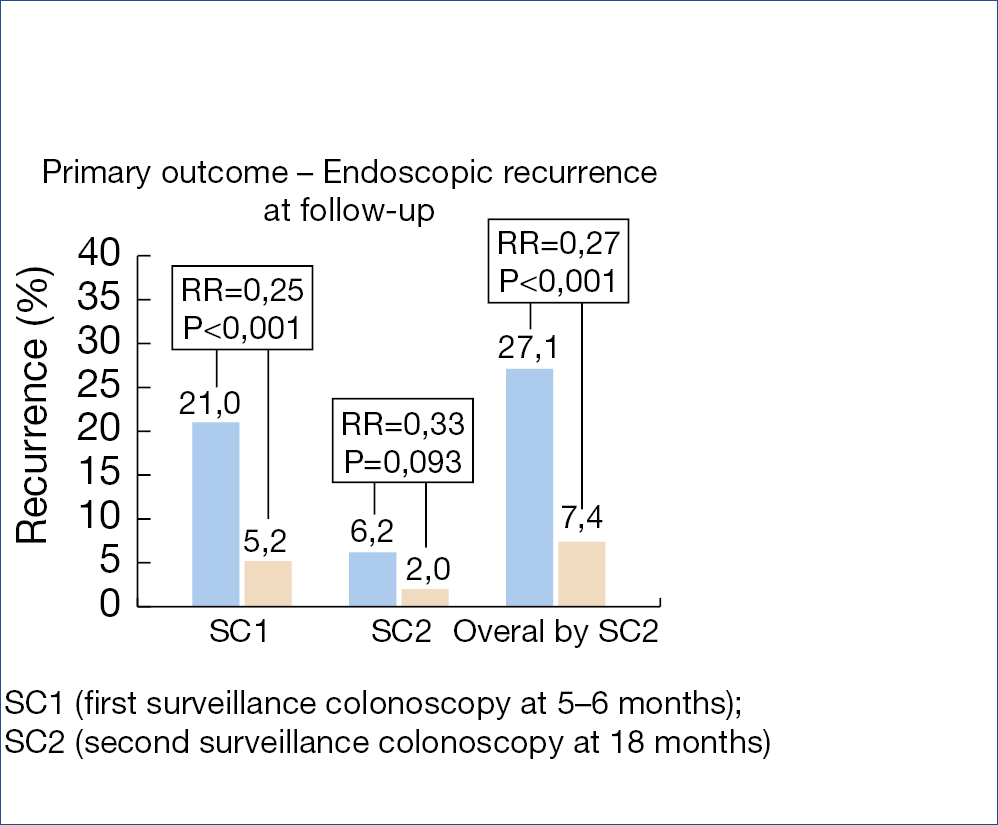
Figure 11.: ADR improvement with AI
• Multicenter RCT (1:1) CADe system vs. control
• 685 patients, 40-80 yrs, diagnostic/screening
Novel pancreatic developments – Peter Layer (Germany)
Obesity, particularly visceral obesity as estimated by measuring girth width, is a significant risk factor for a severe course of acute pancreatitis. A meta-analysis, evaluating some 10.000 patients revealed that a BMI >30 carried a 3-fold higher risk for organ complications and mortality. Peri-pancreatic visceral fat necrosis and lipotoxicity generates a massive release of cytokines and chemokines via adiponectin and unsaturated fatty acids and other toxic compounds, contributing to pulmonary, cardiovascular and renal organ failure. Also, pre-existent diabetes substantially raises the risk for locoregional complications, renal failure and mortality as shown in a meta-analysis involving 355.000 patients with acute pancreatitis. The mechanisms involved remain utterly complex and insufficiently understood.
In previous highlights, the paradigm switch from open necrosectomy to step-up strategy in the management of infected necrotizing pancreatitis has been described and has been accepted as standard therapy in many countries. Initial/early surgery with necrosectomy is associated with a clearly poorer outcome compared with conservative management with individually-adapted step-up strategy: – conservative, where possible; – percutaneous drainage, where necessary; – endotherapeutic debridement, where feasable; – minimally invasive surgical necrosectomy. This overall strategy was confirmed in a recent American retrospective cohort comparing initial open surgical necrosectomy versus a step-up strategy, showing a decrease in mortality from 17% to 6% and a 90-day mortality from 22% to 9%. Also, minimally invasive surgery has been compared to endotherapy in a randomized trial with 66 patients with infected necrosis. Combined mortality and severe complications decreased from 73% to 44% and fistulas from 28% to 0%. The evolution of sirs is shown in the figure below. (Figure 12.)
The beneficial effects of a step-up approach for acute pancreatitis with infected necrosis have also been evaluated after a follow-up period of 86 months in the Dutch trial, comparing open necrosectomy versus step-up. Longterm combined mortality and severe complications decreased from 73% to 44%, incisional hernias from 53% to 23%, pancreatic exocrine insufficiency from 56% to 29% and diabetes from 64% to 40%. There were no significant differences with respect for need for re-intervention, recurrent acute pancreatitis and chronic pain. Based upon all the available studies, we may confidently state that a conservative step-up approach is the current strategy of choice.
Previous highlights stressed the importance of early cholecystectomy in patients with biliary pancreatitis. This was confirmed in the analysis of an American national database of over 40.000 patients with biliary pancreatitis of whom 53% were treated with early cholecystectomy. Relapse of acute pancreatitis within 30 days after onset of biliary pancreatitis dropped from 15% to 6.5% with early cholecystectomy. It is now well documented that patients with biliary pancreatitis should receive early cholecystectomy preferably during the same initial hospitalisation or whenever early feasable.
The clinical manifestations of chronic pancreatitis are quite variable, presenting mainly as bouts of acute inflammation, or progressive fibrosis, duct obstruction and calcification causing persistent abdominal pain, or chronic parenchymal destruction with diabetes and malabsorption not only of lipid soluble vitamins, but also of minerals such as zinc and magnesium. It would appear that alcohol and smoking are the main drivers of the variability of the clinical outcome as illustrated in a large Scandinavian study involving over 1.000 patients (55% alcohol; 53% smoking; 36% combination). Alcohol was mainly involved in the dominant inflammatory phenotype whereas smoking was dominant in the fibrotic and parenchymal destructive phenotype. Thus the individual pathomechanisms (alcohol and / or smoking) are major determinants of the phenotypic clustering of the disease manifestations.
As published in earlier highlights, there is a subgroup of autoimmune pancreatitis patients that does not respond satisfactorily to therapy with corticosteroids and /or immunosuppressants, but does respond to rituximab (anti B-lymphocyte) within over 80% initial complete and in ~50% long-term remission. Whether maintenance rituximab therapy is indicated after the induction therapy to further reduce the relapse rate was retrospectively analysed in 43 patients with a follow-up of ~30 months. The relapse rate dropped from 45% to 11% with maintenance therapy but the clinically-relevant infection rate rose from 0% to 21% respectively. Any therapeutical decision is confronted by the challenging ‘beneficial/adverse‘ balance, stressing the need for individualised therapeutic decisions.
As pancreatic cancer is rising and precursor lesions such as IPMN (intraductal papillary mucinous neoplasia) become increasingly important, it seems justified to summarize the consensus management strategy, based upon worrisome features and high risk features, published in Gut and illustrated below. (Figure 13.)
There is some controversy whether continued surveillance is necessary after IPMN resection. A Japanese study followed 179 patients for 5 years after main duct IPMN resection, of which 14% had HG-dysplasia and 23% cancer. In the pancreatic remnant, malignancy developed in 12%, mainly in those who had cancer in the initial IPMN resectate. These data support the view that continued postoperative surveillance is warranted, especially after resection of IPMN with HG-dysplasia or cancer.
Pancreatic cancer is steadily increasing and is now the third cause of all cancer deaths. Currently, there is no basis for screening the general population but screening is recommended for individuals with increased risk: familial risk or mutations of BRCA1/2, P16/CDKN2A, STK11/LKB1, PRSS1. Surveillance is usually done by alternating yearly EUS and MRI from the 50th year on or 10 yrs before the age of the youngest afflicted relative.
Because the prognosis of symptomatic pancreatic cancer remains dismal, there is rising interest in detecting potential early alarm signs such as idiopathic acute pancreatitis, incidental suspicious imaging findings, incidental elevated lipase, new onset of diabetes mellitus and alteration in the pattern of pre-existing diabetes. An incidentally detected raised lipase, combined with symptoms suggesting a pancreatic disorder or persisting after 4 weeks follow-up requires MRI/EUS examination. If no abnormalities are found, no further surveillance is waranted for this ‘benign hyperenzymemia’. Perhaps clinically more important is the development of ‘new onset of diabetes’ as an early sign of pancreatic cancer, especially when associated with weight loss. Occult diabetes may precede overt pancreatic cancer detection up to 3 years. The cause of the hyperglycemia may well be a paraneoplastic phenomenon, related to excessive cytokine? or adrenomedullin? release, leading to insulin resistance. Interestingly, in some 40% of such patients, the diabetes disappears after resection of the tumor. Also, long-standing pre-existent diabetes raises the risk for pancreatic cancer, and particularly unexplained instable hyperglycemia and weight loss should alert the clinician to the need for detailed imaging studies.
Figure 12.: AP With Infected Necrosis:
Minimally Invasive Surgery vs. Endotherapy
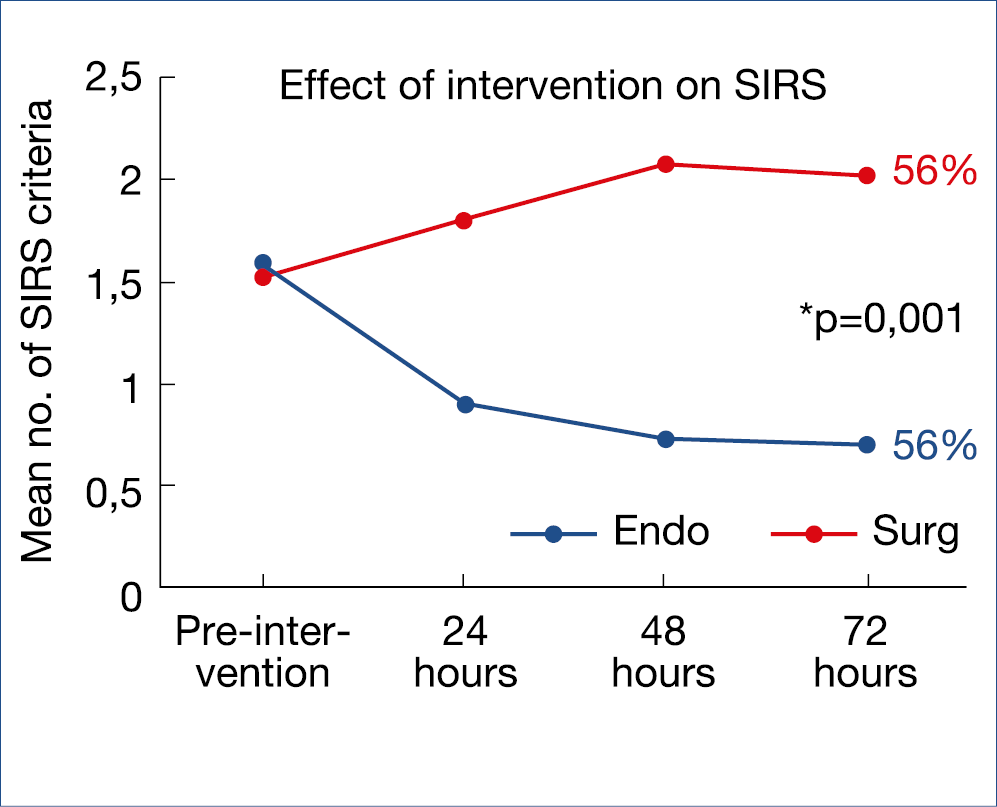
Figure 13.: State of the Art Management of IPMN
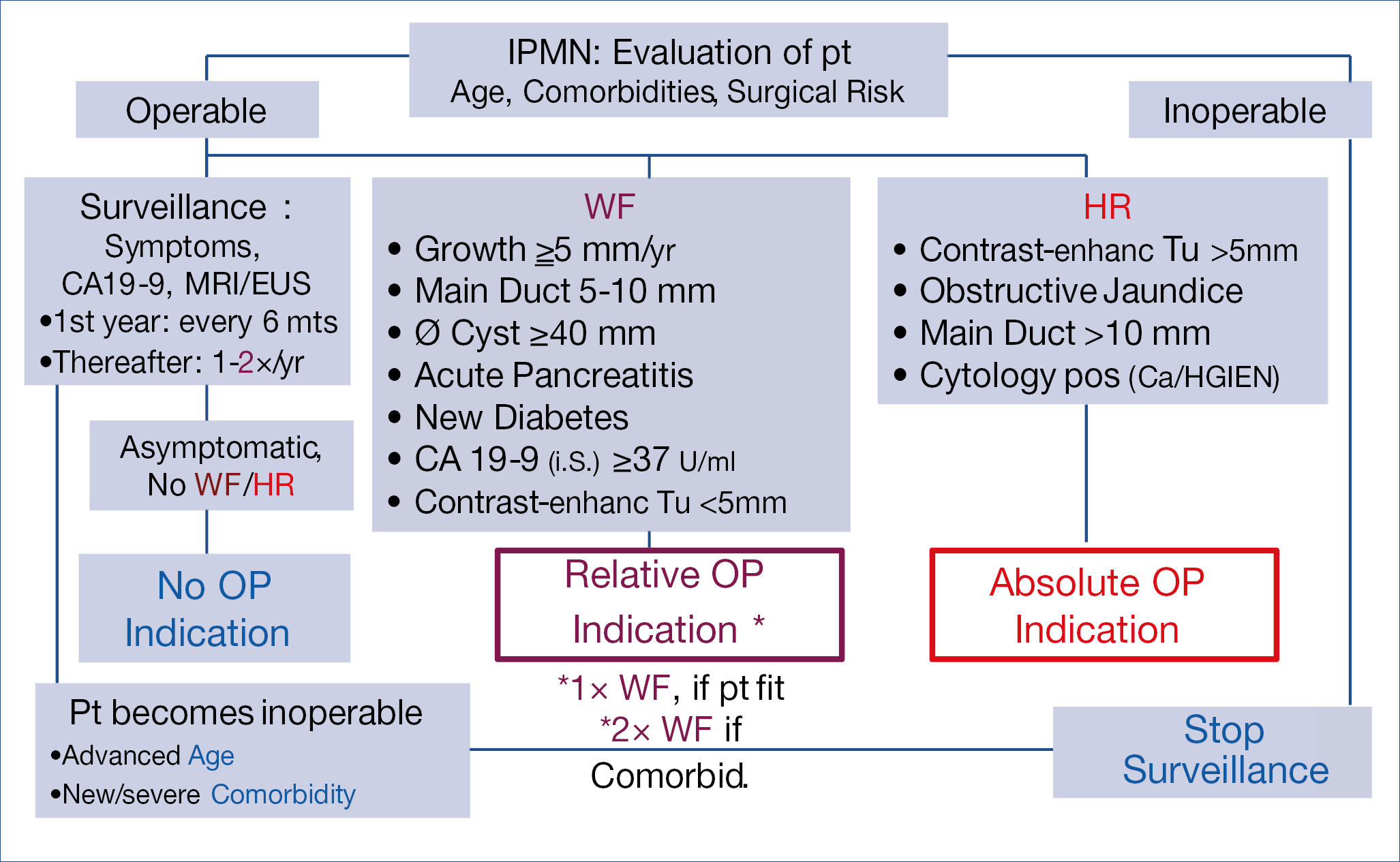
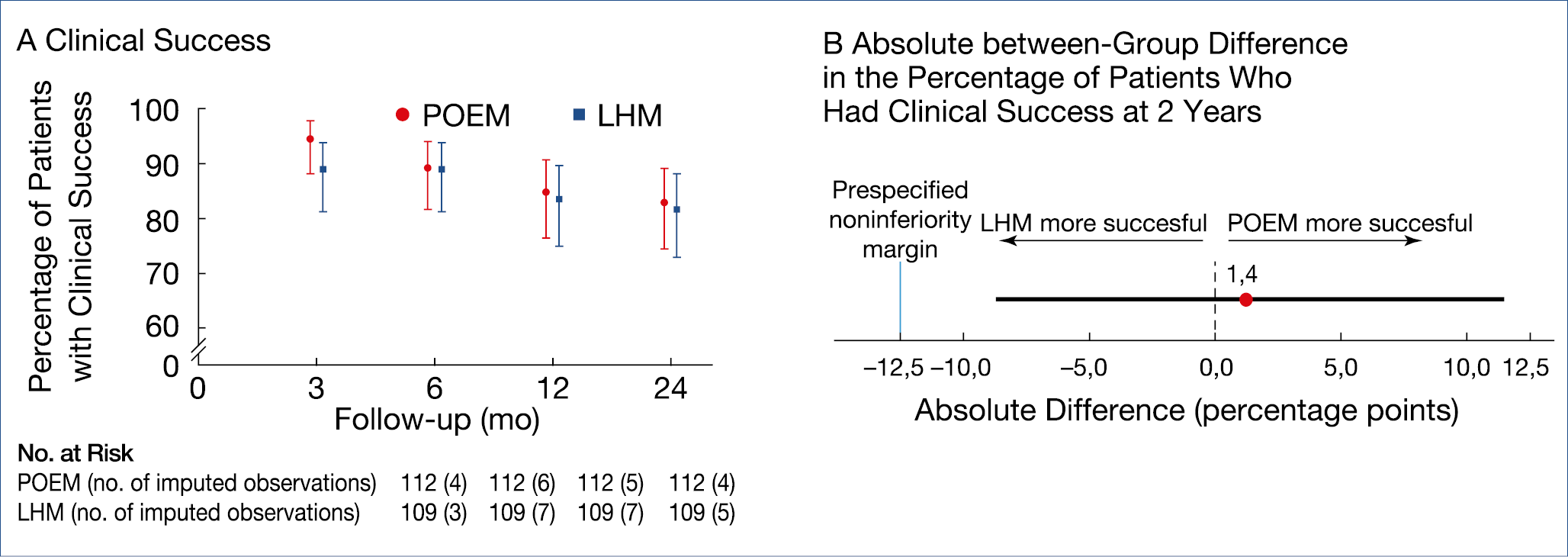
Figure 14.: Achalasia: Peroral endoscopic myotomy vs. surgery
Novel developments in motility and functional disorders – Jan Tack (Belgium)
There are now 3 roughly comparable methodologies for the treatment of esophageal achalasia: pneumatic dilatation; (laparoscopic) Heller myotomy; peroral endoscopic esophageal myotomy (POEM). A recent controlled trial of 43 patients showed that surgery was superior to a single pneumodilation during a 10-year follow-up but a previous large European trial, in which one repeat dilation was allowed, showed equivalent results. POEM has now also been compared to surgery in a multicenter trial, involving over 200 patients. (Figure 14.)
The main difference between endoscopic vs. surgical myotomy was the 44% incidence of reflux esophagitis after POEM, compared to 29% after surgery.
Functional dyspepsia is, according to the Rome IV definition subdivided in – postprandial distress syndrome (PDS) and – epigastric pain syndrome (EPS). Prokinetic drugs do play a role, although modest, in the therapy; as summarized below the proposed foregut effects of the pro-motility drugs do differ. (Figure 15.)
A systematic review of some 30 trials, often of rather somewhat inferior quality, revealed symptom improvement of close to 20% with a NNT of 7. Overall, these results are rather sobering. New is a formulation of microspheres of menthol and caraway oil, previously shown to have analgesic effects. This was evaluated in a controlled trial involving 95 dyspeptics which showed led to rapid symptom relief, especially in PDS patients. Will this drug be useful for intermittent use, if well tolerated?
Gastroparesis, or delayed gastric emptying, in the absence of mechanical obstruction, measured by scintigraphy or breath testing, and occurring with or without diabetes remains a difficult therapeutic challenge. Essentially, only in prokinetic studies where an optimal emptying test was used could symptom improvement in parallel with gastric emptying improvement been shown. New is a recent controlled cross-over trial with 2 mg/d of prucalopride (5HT4 agonist) which showed a clear reduction of the cumulative meal-related symptom score. New is also the evaluation of the neurokinin 1 (NK1) receptor antagonist tradipitant, evaluated in 152 gastroparesis patients, showing significant improvement in nausea severity score and other gastroparesis core symptoms.
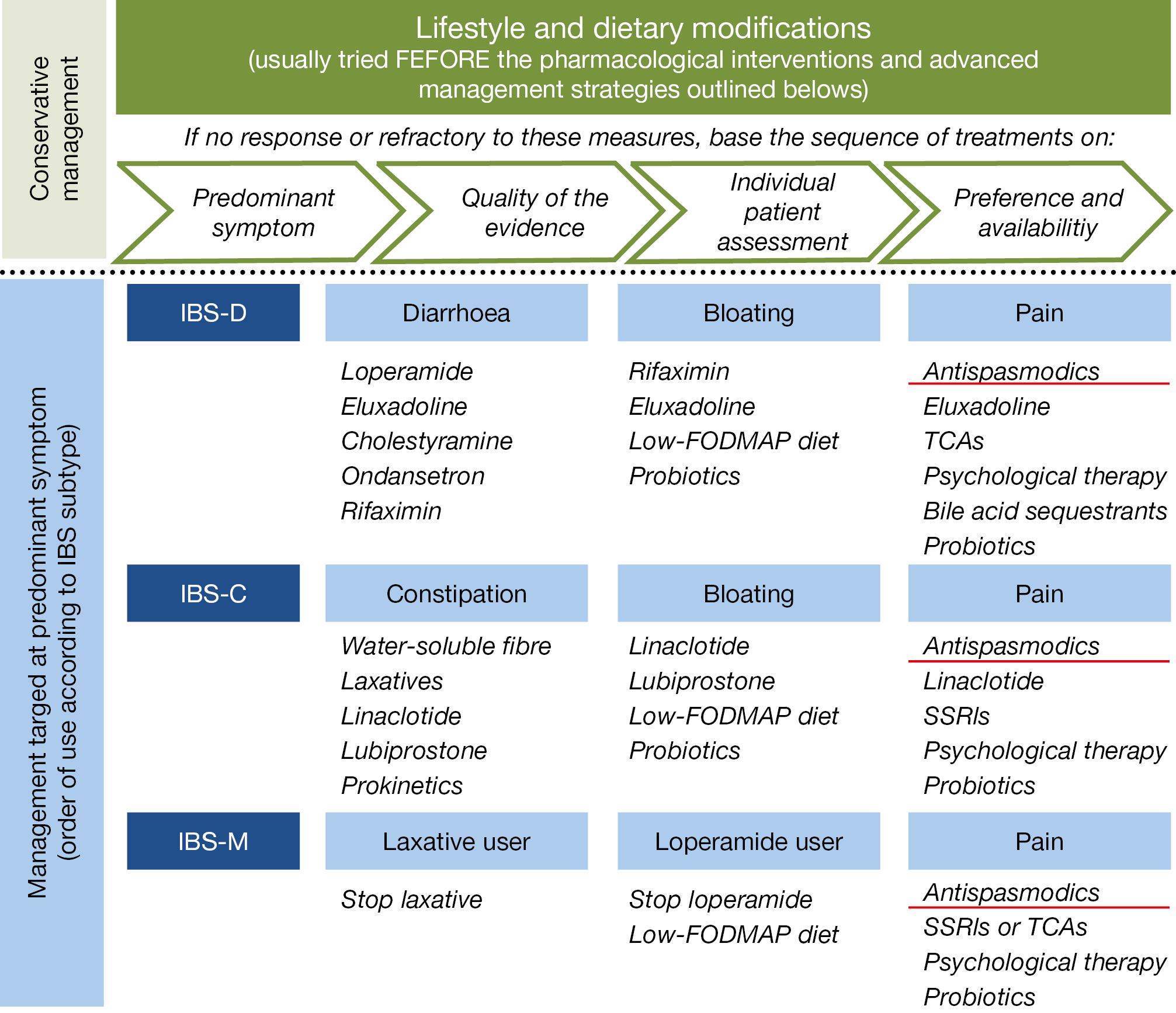
These results are comparable to previous outcome data with aprepitant, indicating that symptomatic control of nausea and vomiting may be another approach to treat gastroparesis. The previously published overall management algorithm of irritable bowel syndrome (IBS), shown in the figure below, is in essence still valid. (Figure 16. ) Treatment with peppermint oil was recently shown to induce moderate improvement of abdominal pain when used in a small intestinal, but not in an ileocolonic release form. A bimodal release form of ondansetron (5HT3 antagonist) was evaluated in 127 diarrhea predominant IBS patients, showing improvement in stool consistency and abdominal pain. A systemic review of several low-FODMAP diet studies revealed an overall benefit in some 31% of IBS patients.
Worth mentioning is the intriguing finding that 70% of IBS patients may display an acute reaction to luminal food allergen challenge, with wheat as the main trigger. This was nicely detected by using mucosal endomicroscopy showing damage and leakage of IV administered fluoresceine after allergen challenge. This was accompanied by signs of eosinophilic infiltration and mucosal barrier impairment. An exclusion diet, based upon such endoscopic evaluation may perhaps have the potential of providing a therapeutic advice. This study indicates again that considering IBS only as a so-called ‘functional’ disorder becomes untenable. We are just at the beginning of disentangling the complexities of allergic, microbial, barrier, hormonal, immune and neuronal -related alterations as the organic substrate of such ‘functional’ disorders. More than ever, ambitious interaction between basic scienentists and clinical investigators is mandatory to advance our discipline, the only way forward to further personalized patient care.
These narrative highlights cover only part of the lectures presented at the Gastro Update Europe 2020 virtual meeting. Not covered in this review are hepatology, oncology, neuromotility, surgery and the exciting case presentations. These highlights intend to illustrate the high educational level of the meeting, emphasizing the most important developments in gastroenterology – hepatology of relevance for the practicing specialist. This type of compact and comprehensive update can be recommended for both fellows and astute practitioners in our discipline.
Footnote: Meeting report of Gastro Update Europe 2020, September 4–5, 2020. This meeting report has been or will be published in full or in part in the following journals: Gastroenterologie a hepatologie, Central European Journal of Gastroenterology and Hepatology, Arab Journal of Gastroenterology, Experimental and Clinical Gastroenterology and Gastroenterologia Kliniczna.
About Gastro Update Europe
The 7th edition of this CME-certified event took place in September, updating delegates on recent developments across the broad spectrum of gastroenterology.
European leaders, whose expertise lie in areas, such as endoscopy, hepatology, small bowel diseases, oncology and much more, spent two compact days summarizing international literature and presenting the most recent cutting-edge developments. The scientific program and speakers were determined by the Scientific Board: Peter Layer (Israelitisches Krankenhaus, Hamburg, Germany), Jaroslaw Regula (Institute of Oncology, Warsaw, Poland), Jan Tack (University Hospital, Leuven, Belgium), and Guido Tytgat (Amsterdam UMC, Amsterdam, Netherlands).
In particular, trial and study results published in peer-reviewed journals during the past year were discussed and practical, relevant take-home messages were presented, with the goal of impacting daily patient care in a positive way.
Gastro Update Europe 2021 – June 11 & 12, 2021
www.gastro-update-europe.eu
Presentations Reviewed:
• Professor Peter Malfertheiner (Oesophagus/Stomach/Duodenum)
Figure 15.: Functional dyspepsia: Efficacy of prokinetics
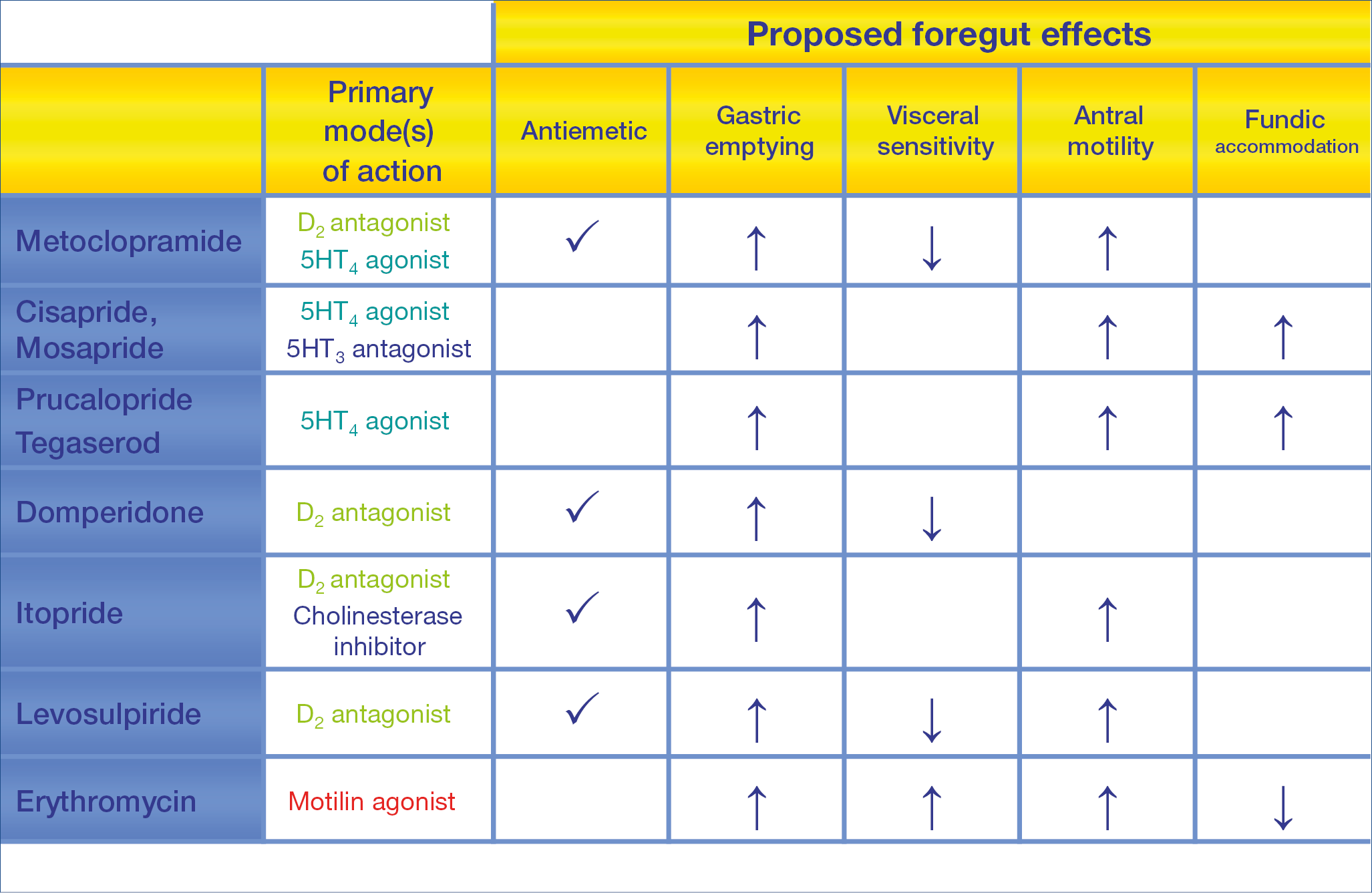
Figure 16.: Irritable bowel syndrome: Management algorithm
University of Magdeburg – Magdeburg
peter.malfertheiner@medizin.uni-magdeburg.de
• Professor Gerhard Rogler (Small Bowel Diseases and
GI Infections)
University Hospital – Zurich
gerhard.rogler@usz.ch
• Professor Jaroslaw Regula (Large Bowel)
Institute of Oncology – Warsaw
jregula@coi.waw.pl
• Professor Oliver Pech (Endoscopy II: Upper & Novel GI Developments)
St. John of God Hospital – Regensburg
oliver.pech@t-online.de
• Professor Marco Bruno (Endoscopy I: EUS, ERCP)
St. John of God Hospital – Regensburg
m.bruno@erasmusmc.nl
• Professor Michal Kaminski (Endoscopy III: Lower GI)
Institute of Oncology – Warsaw
mfkaminski@coi.waw.pl
• Professor Peter Layer (Pancreas)
Israelitisches Krankenhaus – Hamburg
p.layer@ik-h.de
• Professor Jan Tack (Motility & Functional Disorders)
Leuven University Hospital – Leuven
jan.tack@med.kuleuven.be
